
CancerNetwork® spoke with Rafael Fonseca, MD, about the rationale for a simulation using real-world data sets to compare the utility of daratumumab-containing regimens as either frontline or second-line therapy.

Your AI-Trained Oncology Knowledge Connection!


CancerNetwork® spoke with Rafael Fonseca, MD, about the rationale for a simulation using real-world data sets to compare the utility of daratumumab-containing regimens as either frontline or second-line therapy.
Luciano Costa, MD, PhD, spoke about how the results in the final primary analysis of the MASTER trial with daratumumab, carfilzomib, lenalidomide, and dexamethasone in multiple myeloma were not what he expected.

As the year 2021 comes to a close, CancerNetwork® sat down with Yael Cohen, MD, to discuss practice-changing clinical research in multiple myeloma that read out in 2021 and potential FDA updates that may take place in 2022.
The decision by the FDA to approve prostate cancer imaging product TLX591-CDx, a kit to aid in the preparation of gallium-68 gozetotide injections, should make 68Ga-based PSMA-PET more broadly accessible.
Nina Shah, MD, discusses the current treatment options for treating patients with transplant-ineligible multiple myeloma.

Patients with advanced rectal cancer who were treated with up-front chemoradiotherapy plus consolidation chemotherapy experienced an improvement in pathological complete response without impacting disease-free survival.
Susan M. O’Brien, MD, on treating patients with 17p deletions and TP53 mutations who have chronic lymphocytic leukemia.
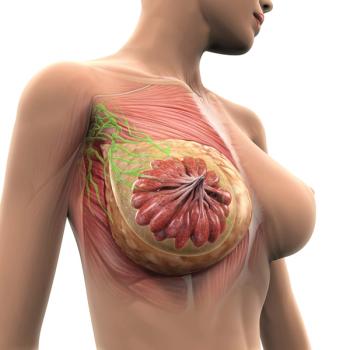
Patients with HER2-mutant breast cancer given neratinib combinations saw improved efficacy.

Nina Shah, MD, on the current treatment options for treating patients with transplant-eligible multiple myeloma.

Paolo Tarantino, MD, hosted a Twitter Takeover during the San Antonio Breast Cancer Symposium where he discussed abstract presentations and key takeaways in a #CNRealTimeReport.

At ASH 2021, CancerNetwork® spoke with Ruben Mesa, MD, of UT Health San Antonio MD Anderson Cancer Center, about big breakthroughs in the treatment of myelofibrosis over the preceding year.
Veteran survivors of head and neck cancer who experienced suicidal self-directed violence were found to most likely die from their injuries.
Steven Devine, MD, spoke about which abstracts he found most interesting at ASH 2021.
CancerNetwork® takes a deep dive into ongoing clinical research that holds promise in the prostate cancer space as the year 2021 comes to a close.

Susan M. O’Brien, MD, on the approved BTK inhibitors for treating chronic lymphocytic leukemia.
Sattva S. Neelapu, MD, spoke about continuing to look at results from the ZUMA-5 trial to determine if axicabtagene ciloleucel can cure patients with relapsed or refractory indolent non-Hodgkin lymphoma

Patients with recurrent ovarian cancer achieved a greater survival benefit after undergoing cytoreductive surgery plus chemotherapy compared with chemotherapy alone.
NUV-422 has demonstrated favorable blood-brain barrier penetration in patients with high-grade gliomas.

In episode 3 of a 4-part small cell lung cancer podcast series, Wade Iams, MD, highlights practical considerations for treating patients with small cell lung cancer, including managing toxicities and the impact of treatment on a patient’s quality of life.

Nina Shah, MD, discusses the biggest news from the FDA for multiple myeloma in the past year.

Patients with triple-class relapsed/refractory multiple myeloma treated with ciltacabtagene autoleucel saw a greater survival benefit over physician's choice of treatment.

Abatacept can now be used for the prevention of acute graft-versus-host disease following its approval by the FDA.

Most subgroups of patients with relapsed or refractory, heavily pretreated multiple myeloma showed durable responses at the 2-year follow-up to the CARTITUDE-1 trial.

Patients with heavily pretreated relapsed/refractory multiple myeloma achieved promising benefit in terms of cytokine release syndrome mitigation via double cycle 1 step-up dosing for cevostamab.

Deep responses were seen with single infusion ciltacabtagene autoleucel for heavily pretreated patients with multiple myeloma who were refractory to lenalidomide.

When asked about abstracts he thinks have the greatest potential to impact the standard of care in myeloma, Rafael Fonseca, MD, looked to emerging cellular therapies, specifically ciltacabtagene autoleucel for the treatment of heavily pretreated disease.

CancerNetwork® looks back on some of the promising, ongoing pieces of clinical research within the breast cancer space from 2021.
Second-line treatment of lisocabtagene maraleucel demonstrated improved quality of life in patients with large B-cell lymphoma.

Maintenance oral azacitidine produced a sustained survival benefit over placebo for patients with acute myeloid leukemia in first remission.

Susan M. O’Brien, MD, discussed the emergence of noncovalent BTK inhibitors for treating chronic lymphocytic leukemia.