
Data from the COMBI-AD Trial evaluating dabrafenib and trametinib to treat melanoma was analyzed by Hussein Tawbi, MD, PhD, of The University of Texas MD Anderson Cancer Center.

Your AI-Trained Oncology Knowledge Connection!


Data from the COMBI-AD Trial evaluating dabrafenib and trametinib to treat melanoma was analyzed by Hussein Tawbi, MD, PhD, of The University of Texas MD Anderson Cancer Center.

CancerNetwork® will be hosting a tweet chat this Monday, November 16th, at 12 PM EST, that will feature real patient cases regarding the use of integrative oncology.

Investigators suggested that guidelines which recommend initiating screening mammography at age 50 may be putting Black women at a disadvantage, and screening should thus begin by age 40.

This study found that deaths from cancer accounted for more than 4 million potential years of life lost in 2017.

CancerNetwork spoke with Jason Luke, MD, FACP, at the SMR Conference regarding the latest updates on melanoma.
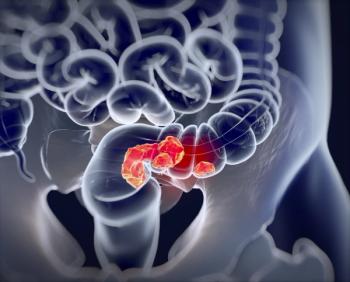
Watch-and-wait in place of surgery was shown to be a safe and feasible treatment approach for patients with nonmetastatic rectal cancer achieving a complete response to neoadjuvant chemoradiation.

Researchers indicated that there is still a persistent survival disparity that has not narrowed over 2 decades between white and Black patients with inflammatory breast cancer.

The Cancer Experience Registry was developed by the Cancer Support Community in order to collect information about the emotional and social effects of living with a cancer diagnosis.
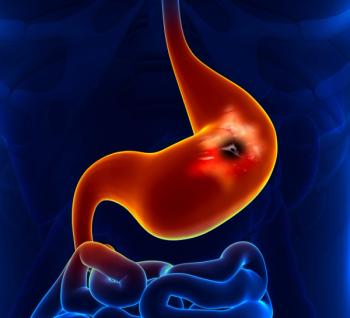
“These results bring us one step closer to the first potential targeted therapy for advanced gastric cancer in over a decade,” said Helen Collins, MD.

The final guidance provides the agency’s current thinking regarding steps to broaden eligibility criteria in clinical trials through inclusive trial practices, trial designs, and methodological approaches.

The latest installment of “Oncology Peer Review On-The-Go” dissects original research published in the journal ONCOLOGY regarding potential barriers to clinical trial enrollment for patients with pancreatic cancer.
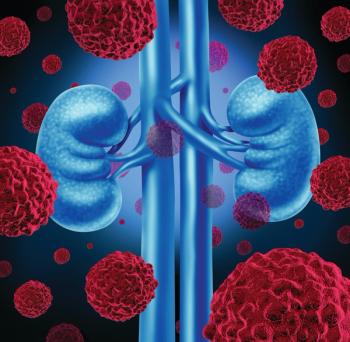
The phase 3 KEYNOTE-581/CLEAR trial (Study 307) demonstrated that both combinations saw meaningful improvements in the study’s primary end point of progression-free survival for patients with advanced renal cell carcinoma.

Merck is discontinuing the study after an independent Data Monitoring Committee recommendation, which determined the benefit/risk profile of pembrolizumab in combination with ipilimumab did not support continuing the trial.

“The data reported, in addition to its ease of use, demonstrate the potential of [tavokinogene telseplasmid] in combination with pembrolizumab as a next-generation intratumoral IL-12 therapy that can induce regression of both locally treated and untreated distant and visceral lesions,” said Paolo A. Ascierto, MD.

The survey highlighted the need for further research into the specific effects of ITP in individual patients so that these findings can be better integrated into the management of this patient population.
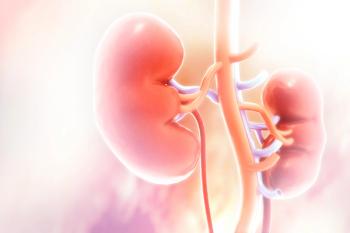
The results of the phase 2 Study 218 indicated that the lower starting dose of 14 mg of lenvatinib (Lenvima) did not meet the threshold for non-inferiority compared to the FDA-approved starting dose of 18 mg.

The FDA has approved the FoundationOne Liquid CDx to identify patients with BRCA1, BRCA2, and/or ATM alterations in patients with metastatic castration-resistant prostate cancer who may be appropriate for treatment with olaparib.

Jeffrey Schneider, MD, and Marc Braunstein, MD, PhD, spoke about their recent article published in the journal ONCOLOGY® regarding the management of patients with cancer during the COVID-19 pandemic.
Results from this study suggest that this combination is active and should be considered for this patient population, though additional research is still necessary.

A recent study found that automated analyses of CT scans for patients with breast cancer can predict which patients are likely to develop cardiovascular disease in the future.
This study suggested that there is evidence, though low quality, that rituximab (Rituxan) may be a more effective second-line therapy than splenectomy for children with immune thrombocytopenia.
For adult patients with relapsed or refractory T-cell acute lymphoblastic leukemia/T-cell lymphoblastic lymphoma, a phase 1 clinical trial revealed that crenigacestat (LY3039478) demonstrated little clinical activity at the recommended dose.

AstraZeneca and Merck announced that the combination treatment of olaparib with bevacizumab to treat adult patients with advanced high-grade epithelial ovarian, fallopian tube or primary peritoneal cancer was approved in the European Union.

Patients with immune thrombocytopenia treated with rituximab (Rituxan), splenectomy, eltrombopag (Promacta), or romiplostim (Nplate) saw significant increases in platelet count with each of the 4 treatments.
A study presented at the 12th European Breast Cancer Conference suggests women over the age of 70 with breast cancer can tolerate surgery even though they aren’t offered it regularly, but a second abstract suggests these women tend to opt out of this treatment option.

The results for the phase 2 SUMMIT trial examining neratinib to treat patients of metastatic non-small cell lung cancer (NSCLC) were recently announced by Puma Biotechnology.
The study suggested that voriconazole (Vfend) may be the best prophylaxis option for patients undergoing HSCT, and posaconazole (Noxafil) may be the best prophylaxis option for patients with AML or MDS.
In a mock tumor board on prostate cancer on Twitter, health care providers came together to discuss real patient cases and present various treatment options for review.
In working patients with head and neck cancer receiving radiotherapy, weight loss and an increase in pain, fatigue, and ECOG status were all found to be significantly associated with reductions in working days per week.
This study suggested that the racial composition of clinical trials involving radiation therapy does not match that of the overall US population.