
Outcomes favoring robotic surgery for CRC may be influenced by patient selection factors, including clinical stability.

Your AI-Trained Oncology Knowledge Connection!


Outcomes favoring robotic surgery for CRC may be influenced by patient selection factors, including clinical stability.
Data suggest that sotorasib plus panitumumab may represent a valuable new treatment option in this KRAS G12C–mutated colorectal cancer population.

Geraldine O’Sullivan Coyne, MD, PhD, MRCPI, described the excitement of seeing novel molecules such as antibody-drug conjugates become more prominent.

Results from a retrospective study showed that chemoimmunotherapy did not prolong survival vs chemotherapy in a younger ES-SCLC population.

Findings from the KRYSTAL-12 trial support adagrasib as a treatment option for those with disease progression on prior chemotherapy and immunotherapy.

Ronald Bleday, MD, credits a chronic pain clinic for consulting patients who may be at a greater risk for prolonged opioid use following surgery.
Results from the SunRISe-1 trial showed that TAR-200 monotherapy achieved a complete response rate of 82.4% in patients with BCG-unresponsive NMIBC.

Results from the phase 3 KEYNOTE-689 trial supported the agency’s approval of the pembrolizumab/surgery regimen in PD-L1–positive head and neck cancer.
An update of phase 1 data from the CTMX-2051-101 study is expected to be available by the first quarter of 2026.

The primary end points of the EPCORE FL-1 trial were met while assessing an epcoritamab-based combination for patients with R/R follicular lymphoma.

Patients with extensive-stage small cell lung cancer experienced a median PFS and OS of 4.5 months and 7.2 months, respectively, with anlotinib plus irinotecan.
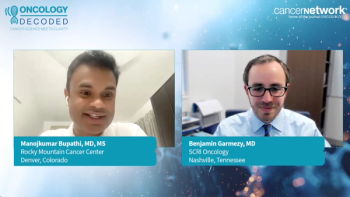
Oncology Decoded provides the current management of metastatic bladder cancer, recent advancements in systemic therapy, patient selection, and AE mitigation.

Tran Ho, DO, FSSO, FACS, discussed how she found her passion in breast surgical oncology and how her mentors helped her achieve success.

Geraldine O’Sullivan Coyne, MD, MRCPI, PhD, shares how a new position presents a “good opportunity” to improve community-based clinical trial access.

The latest in first- and second-line therapies for bladder cancer, including trial data, AE management, and the role of molecular testing, were discussed in the latest Oncology Decoded podcast.

Developers are enrolling those with metastatic castration-resistant prostate cancer on a phase 1/2 trial to assess the safety and tolerability of HLD-0915.

The development of nonnarcotic pain medication after GI surgery may help relieve chronic pain without the risk of opioid dependence.

Ronald Bleday, MD, stated that before standardizing a stepwise approach to treating surgical pain, providers might have overtreated patients with opioids.

FLEX study findings show that the MammaPrint Index was predictive of 5-year DRFI for endocrine therapy with or without chemotherapy in early breast cancer.

Additional research on novel targeted therapies may be necessary to address the unmet needs in this high-grade serous ovarian cancer population.
The CD19 t-haNK therapy alone and in combination with rituximab achieved complete responses and no significant toxicities in 2 patients with late-stage WM.

The drug that the companion diagnostic identifies patients for, zongertinib, received FDA approval for HER2-mutant NSCLC on August 8, 2025.

Conducting trials safely within a community setting lies at the heart of a successful collaboration between Northwell Health and START.
Patients with cisplatin-ineligible bladder cancer who received enfortumab vedotin plus pembrolizumab and surgery had prolonged survival vs those who received surgery alone.

The novel cancer vaccine plus pembrolizumab missed statistical significance but generated a clinically meaningful improvement in advanced melanoma.
Findings demonstrate a need to move beyond disease-related factors to address disparities in HMA treatment patterns among those with MDS.

The expertise of START's network may streamline the availability of clinical trial enrollment and novel treatment options among patients with cancer.

The decision is based on phase 3 VERITAC-2 trial data showing a significant PFS improvement vs fulvestrant in ESR1-mutant ER+/HER2– breast cancer.

A panel of clinical pharmacists discussed strategies for mitigating toxicities across different multiple myeloma, lymphoma, and leukemia populations.

A new START center in New York may give patients with advanced malignancies an opportunity to access novel therapies in the community setting.