
More than 80% of patients in cohort B of the QUILT-3.032 study avoided cystectomy at 36 months after treatment with nogapendekin alfa plus BCG.

Your AI-Trained Oncology Knowledge Connection!


Russ Conroy is an Associate Editor for CancerNetwork. He grew up in Hillsborough, New Jersey, and graduated from Rutgers University-New Brunswick in 2022.
On the weekends, he likes to unwind by playing video games with friends, tailgating at Rutgers football games with his family, or building his music collection with a visit to Princeton Record Exchange.

More than 80% of patients in cohort B of the QUILT-3.032 study avoided cystectomy at 36 months after treatment with nogapendekin alfa plus BCG.

Data from the phase 3 PALOMA-3 study support the approval of subcutaneous amivantamab in this NSCLC population.

Key presentations from the 2025 ASH meeting revealed potential therapeutic advances across leukemia, multiple myeloma, and lymphoma.

Data from the phase 3 AMPLITUDE trial support the approval of niraparib plus abiraterone acetate and prednisone for this metastatic CSPC population.

The COMPASSION-37 study is the second international registrational study for cadonilimab following an ongoing trial in hepatocellular carcinoma.

Findings from the P-RAD trial show encouraging rates of pathologic complete response among patients who received pembrolizumab plus radiotherapy.

Data from the phase 1b MonumenTAL-2 study support continued investigation of talquetamab/pomalidomide in the phase 3 MonumenTAL-6 trial.

Higher ECOG performance statuses and metastatic burden predicted poorer outcomes with frontline adebrelimab for those with extensive-stage SCLC.

Across different studies, the highest incidence of left ventricular ejection fraction decrease was seen with trastuzumab/pertuzumab plus chemotherapy.

More than half of evaluable patients in the phase 1/2 NEXICART-2 trial experienced organ responses following treatment with NXC-201.

Future work may expand analyses of measurable residual disease as a surrogate end point in AML to the use of modern, non-intensive treatment backbones.

Over 80% of a small cohort of patients with mantle cell lymphoma achieved a complete response to ibrutinib plus venetoclax.

Updated results at ASH 2025 may support new alternatives to continuous therapy and standard intensive chemotherapy across different leukemia types.

From phase 3 trial updates to results on trispecific antibodies, ASH 2025 may feature a variety of practice-shifting presentations across multiple myeloma.

Qualified surgeons may offer laparoscopic distal gastrectomy as an alternative to open distal gastrectomy among those with clinical T4a gastric cancer.

Phase 1 data related to CLN-049 for patients with acute myeloid leukemia will be presented at the 2025 ASH Annual Meeting and Exposition.

Findings from the HERIZON-BTC-01 trial support HER2 as a valid therapeutic target in biliary tract cancer.
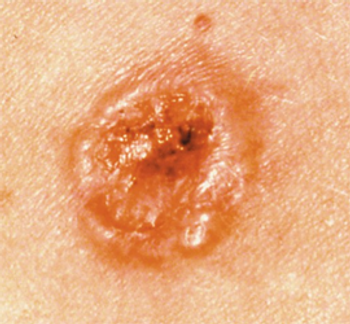
Updated results from the phase 1 CK-301-101 trial support the updated label for cosibelimab in this cutaneous squamous cell carcinoma population.

Investigators noted 1 instance of rectovaginal fistula in the PEG gel arm, although rapid postoperative recovery occurred.

Investigators are currently assessing the safety and preliminary activity of AVZO-103 among patients with advanced solid tumors in a phase 1/2 trial.

One patient with newly diagnosed glioblastoma multiforme reached 3 years of survival following treatment with temferon in a phase 1/2a trial.
Subgroup data from the ARASENS trial support darolutamide plus androgen deprivation therapy as a standard of care in metastatic HSPC regardless of age.
Treatment with MCS-8 yielded no serious adverse effects among patients at a high risk of developing prostate cancer.

Deeper short-term declines in quality of life occurred when combining cisplatin with radiation for those with intermediate-risk cervical cancer.

Pharmacokinetic data from the phase 1/2 GO29781 study support the European approval of subcutaneous mosunetuzumab in this follicular lymphoma population.

Data from the DeLLphi-304 trial support the full approval of tarlatamab in this extensive-stage small cell lung cancer population.

A busy week for the FDA saw notable regulatory approvals across different breast cancer, acute myeloid leukemia, and endometrial cancer indications.

Data show that XCR1-positive conventional type 1 dendritic cells may play a role as mediators of response to atezolizumab in extensive-stage SCLC.
Receiving treatment at an academic center may improve the probability of receiving subsequent curative care among those with hepatocellular carcinoma.

Data from a phase 1/2 trial demonstrate the potential antitumor activity of FOG-001 in patients with desmoid tumors.