
Data from the phase 3 BEACON CRC trial support the approval of encorafenib plus cetuximab for this colorectal cancer population in China.

Your AI-Trained Oncology Knowledge Connection!


Russ Conroy is an Associate Editor for CancerNetwork. He grew up in Hillsborough, New Jersey, and graduated from Rutgers University-New Brunswick in 2022.
On the weekends, he likes to unwind by playing video games with friends, tailgating at Rutgers football games with his family, or building his music collection with a visit to Princeton Record Exchange.

Data from the phase 3 BEACON CRC trial support the approval of encorafenib plus cetuximab for this colorectal cancer population in China.
Use of TumorSight Viz may support improved consistency, precision, and efficiency in breast cancer surgery.
Data from the RATIONALE-309 trial support the European Commission’s approval of frontline tislelizumab plus chemotherapy in this patient population.

Treatment with tarlatamab demonstrated intracranial responses in a real-world cohort of patients with extensive-stage small cell lung cancer.

Investigators of the phase 2 PEVOsq trial identify PD-L1 positivity and HPV positivity as predictive biomarkers of response to the pembrolizumab regimen.

Final data from the phase 3 ENLIVEN trial demonstrate long-term tumor responses and a consistent safety profile with pexidartinib.

Treatment with ISB 2001 demonstrated robust responses across difficult-to-treat multiple myeloma subgroups in the phase 1 TRIgnite-1 study.

Longer follow-up from the phase 3 MIDAS trial may be necessary to evaluate progression-free survival and overall survival.

HAIC with oxaliplatin and raltitrexed produced a higher response rate vs other systemic therapy agents in patients with advanced hepatocellular carcinoma.

Current data support prospective studies comparing IDH triplet vs IDH doublet therapies among patients with IDH-mutant acute myeloid leukemia.

Phase 3 data affirm the use of partial-breast intensity-modulated radiotherapy as a standard of care in patients with low-risk early-stage breast cancer.
Confirmatory data may support zanidatamab as an advancement in the treatment of HER2-positive advanced gastroesophageal adenocarcinoma.

Investigators will present detailed results from the phase 3 FORTITUDE-101 trial at a future medical meeting.

Data from the phase 1/2 WU-KONG1 study support the accelerated approval of sunvozertinib in this population.
Use of the novel artificial intelligence–based test may provide a painless, low-cost alternative in bladder cancer screening.

Data support incorporating volumetric PET biomarkers into toxicity risk prediction for patients receiving CAR T-cell therapy for LBCL.
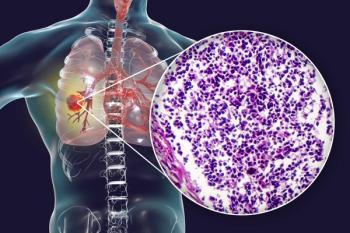
Data from the phase 3 ASTRUM-005 trial support the MHRA’s approval of serplulimab for patients with extensive-stage small cell lung cancer.
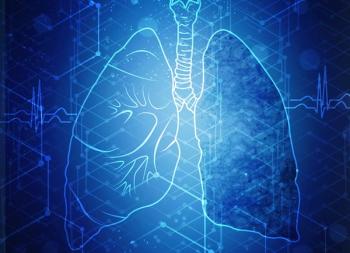
Compared with 18F-FDG, the use of 68Ga-NODAGA-LM3 appears to favor bone and brain lesion detection among patients with small cell lung cancer.

Data from part B of the DeFianCe study demonstrate a positive overall response rate trend with sirexatamab plus bevacizumab and chemotherapy.

The FDA has set a Prescription Drug User Fee Act date of October 25, 2025, for approving revumenib in this acute myeloid leukemia population.

Developers plan to begin a rolling new drug application for zidesamtinib as a treatment for this non–small cell lung cancer population in July 2025.

More than 70% of patients achieve an objective response with isatuximab plus pomalidomide and dexamethasone in the phase 2 EAE115 trial.

Use of PET-guided radiotherapy may enable the opportunity to incorporate biological information into the planning and delivery of radiation.

Investigators will submit detailed results from the phase 3 STELLAR-303 trial for presentation at a future medical conference.

Data from the phase 3 CABINET trial support the CHMP’s positive opinion of cabozantinib in well-differentiated extrapancreatic or pancreatic NETs.

Data from the phase 3 GEMSTONE-302 trial support sugemalimab plus chemotherapy as a frontline treatment option in metastatic non–small cell lung cancer.

Data from the 2025 EHA Congress show developments in novel therapeutic strategies across different multiple myeloma, leukemia, and lymphoma populations.

Low rates of early relapse in both arms of the phase 3 IsKia trial support the efficacy of carfilzomib in this newly diagnosed multiple myeloma setting.

Biomarker analyses indicate the predictive potential of a 15-protein signature related to the efficacy of serplulimab plus chemotherapy in ES-SCLC.

Data from APPULSE-PNH may support oral iptacopan as a potentially practice-changing option in patients with paroxysmal nocturnal hemoglobinuria.