
Researchers at the Johns Hopkins University Bloomberg School of Public Health say they have found several gene variants which may predispose individuals to develop gall bladder cancer.

Your AI-Trained Oncology Knowledge Connection!


Researchers at the Johns Hopkins University Bloomberg School of Public Health say they have found several gene variants which may predispose individuals to develop gall bladder cancer.

Overweight individuals in early adulthood who gained additional weight to become obese later in life are at an increased risk for esophageal and gastric cardia adenocarcinomas.

We spoke with Dr. Luis Diaz about his recent presentation on immunotherapy in colorectal cancer at the 2017 American Society of Clinical Oncology (ASCO) Gastrointestinal Cancers Symposium, held January 19–21 in San Francisco.

In my practice, I am constantly being asked how to properly treat scalp involvement in relation to these skin toxicities.

Ahead of the 2017 ASCO Gastrointestinal Cancer Symposium in San Francisco, we spoke with Dr. Geoffrey Ku on the advances in systemic therapy for esophageal and gastric cancer.

New data are suggesting that deciphering the genomic diversity and evolution of tumors may provide a basis for identifying new targets and designing improved personalized medicine strategies.

The Pancreatic Cancer Action Network is aiming to improve the lives of patients diagnosed with the third-leading cause of cancer-related death with a new precision drug trial.

If you’ve been in oncology long enough, you’ve likely seen the patient who presents with metastatic disease, gets first-line therapy, progresses, switches to second-line therapy, progresses again, and so on, with their cancer becoming increasingly more resistant to therapy.

Circulating tumor DNA detected after resection of stage II colon cancer appears to identify patients who are at high-risk of tumor recurrence.

Here we review the evidence supporting current approaches to resectable gastric cancer, including discussion of the optimal extent of surgery and lymphadenectomy, adjuvant chemotherapy, postoperative chemotherapy with chemoradiation, and perioperative chemotherapy.

Current methods of treatment still have a small impact on the survival of patients with localized disease. Improved understanding of the underlying mutations seen in gastric cancer might suggest alternative treatments and ways to better select patients.

Researchers are reporting they have identified ERCC3 as a new target for pancreatic cancer and demonstrated the effects are extremely strong in a molecularly defined subset of patients.

Lymph node status after undergoing neoadjuvant chemotherapy and resection of esophagogastric cancer was the only independent predictor of survival.

A novel first-in-class antibody can significantly extend median survival when added to standard chemotherapy for patients with advanced gastric cancer.

Combined therapy with trastuzumab and the tyrosine kinase inhibitor lapatinib shows promise against chemotherapy-refractory, HER2-positive colorectal cancers that do not harbor KRAS mutations.
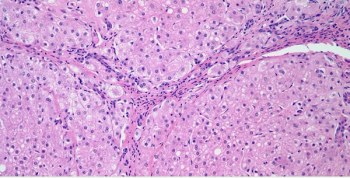
A 35-year-old woman was found to have a 4 cm nodule in the liver. After a biopsy is performed, what is your diagnosis?

For the first time, researchers suggest that underlying genetic mutations may cause pancreatic adenocarcinoma tumors to stiffen up and produce a dangerous thickening and scarring of the tissue around them.

Clinicians now have a new tool to better identify colorectal cancer in its early and more treatable state.

Researchers at Wake Forest Baptist Medical Center’s Institute for Regenerative Medicine are hoping to help advance the war against cancer with a new system called, “metastasis-on-a-chip.”
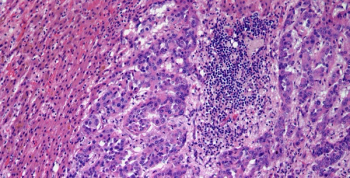
A 53-year-old man presents with a large hepatic mass. After further evaluation, a biopsy is performed. What is your diagnosis?

A meta-analysis found that a number of dietary factors play a role in the risk of developing gastric cancer, with protective effects seen with fruit and certain vegetables and increased risk seen with high-salt foods and certain forms of alcohol.

Treatment with the PARP inhibitor olaparib plus paclitaxel resulted in an overall survival benefit in patients with metastatic gastric cancer.
New data suggests that an experimental targeted therapy known as IMMU-130 may be effective in controlling drug-resistant metastatic colorectal cancer (mCRC) in patients previously treated with irinotecan-containing chemotherapy regimens.
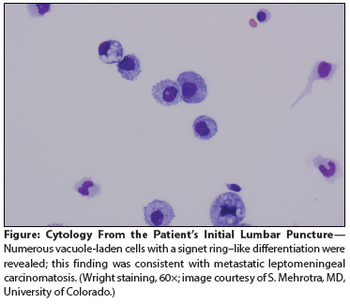
A 59-year-old man with metastatic gastric cancer presented to the oncology clinic with a 1-week history of positional headache, nausea, and vomiting. He stated that the headache was located in the frontal region, was 8 on a scale of 10 in intensity.
According to a large study, men with a CDH1 gene mutation have a 70% incidence of gastric cancer by the age of 80 years and women have a 56% incidence.