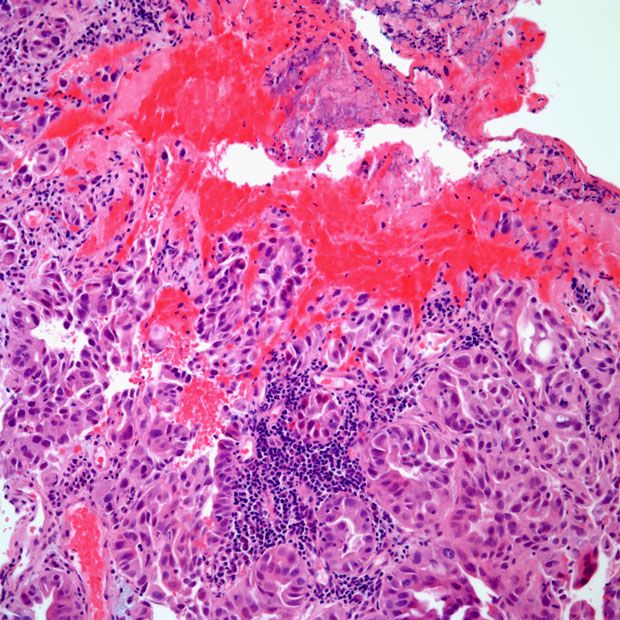
Efficacy and safety findings from the phase 3 GLOW trial assessing zolbetuximab and CAPOX in CLDN18.2-positive locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma were consistent with the phase 3 SPOTLIGHT trial, according to an expert from Weill Cornell Medical College