
A new study from The Cancer Genome Atlas proposes a new classification of gastric cancers into four genomic subtypes that could eventually yield better treatment paradigms.

Your AI-Trained Oncology Knowledge Connection!


A new study from The Cancer Genome Atlas proposes a new classification of gastric cancers into four genomic subtypes that could eventually yield better treatment paradigms.

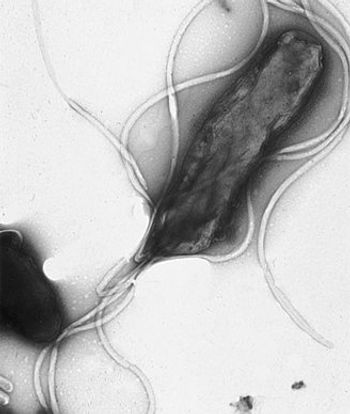
Searching for and eradicating the bacteria Helicobacter pylori could reduce the incidence of gastric cancer in otherwise healthy individuals, especially in Asian populations, according to a new study.

Both esophageal cancer and stomach cancer are aggressive malignancies with contrasting risk factors, histologies, and molecular characteristics-yet for the most part comparable therapeutic approaches.

The purpose of this review is to update, present some of the new data on, and outline the controversies regarding neoadjuvant and adjuvant therapy of esophagogastric junction and gastric adenocarcinoma.
Adding ramucirumab to FOLFOX did not delay progression in patients with untreated advanced gastric or esophageal adenocarcinoma, according to trial results presented at the 2014 ASCO Annual Meeting.

The VEGFR-2 tyrosine kinase inhibitor apatinib significantly prolonged overall survival in patients with advanced gastric cancer compared with placebo, according to the results of a phase III study.

The FDA has approved the angiogenesis inhibitor ramucirumab for the treatment of gastric cancer and gastroesophageal junction adenocarcinoma.

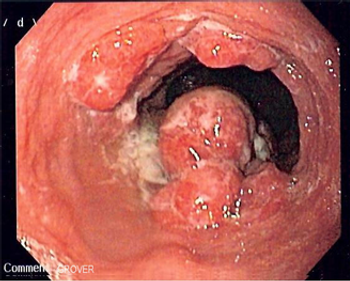
Confocal laser endomicroscopy, a new technology that permits high-resolution subsurface microscopic imaging of living tissue during routine endoscopy, can facilitate the diagnosis of esophageal and gastric cancers, according to a recent report. "Endomicroscopy allows you to make an in vivo histology during ongoing endoscopy," Ralf Kiesslich, MD, PhD, said at the 2006 Gastrointestinal Cancers Symposium (General Session I).

The FDA has approved the use of Taxotere (docetaxel, Sanofi-Aventis) in combination with cisplatin and fluorouracil (5-FU) for the treatment of advanced gastric cancer, including cancer of the gastroesophageal junction, in patients who have not received prior chemotherapy for their advanced disease.

ORLANDO-Chemotherapy given before and after surgery is better than surgery alone for operable gastric and lower esophageal cancers, David Cunningham, MD, reported (abstract 4001).

BEIJING, CHINA-“Capecitabine (Xeloda) plus cisplatin is highly active and well tolerated as first-line treatment of Chinese patients with advanced gastric cancer, with an overall

The 30 reports in this special supplement to Oncology News International represent highlights of ongoing major clinical trials and new research presented at ASCO 2004 regarding state-of-the-art chemotherapeutic management of gastrointestinal and other cancers. Important developments in capecitabine as adjuvant therapy, novel targeted agents, and new combinations are discussed.

This special “annual highlights” supplement to Oncology News International is a compilation of some of the major advances in the management of gastrointestinal cancers during 2003–2004, as reported in ONI. Guest editor Dr. James L. Abbruzzese comments on the reports included herein and discusses advances in the clinical management of GI cancers, with a focus on developments in targeted therapy, new combinations, adjuvant therapy, and what to watch for in 2004.

Docetaxel (Taxotere) has been successfully investigated in the therapy for advanced gastroesophageal tumors as both a single agent and in combination regimens. As a single agent, phase II study results demonstrate an overall response rate of 17% to 24%, with occasional complete responses in a disease in which complete responses are rare. These figures classify docetaxel among the most active agents for the disease.

We conducted a phase II study to assess the response rate and toxicity profile of the irinotecan (CPT-11, Camptosar) plus cisplatin combination administered weekly to patients with at least one previous chemotherapy for advanced adenocarcinoma of the stomach or gastroesophageal junction. Patients with histologic proof of adenocarcinoma of the stomach or gastroesophageal junction with adequate liver, kidney, and bone marrow functions were treated with 50 mg/m² of irinotecan plus 30 mg/m² of cisplatin, both administered intravenously 1 day a week for 4 consecutive weeks, followed by a 2-week recovery period.

Metastatic gastric cancer is a relatively chemosensitive disease. With current regimens, 25% to 40% of patients can be expected to respond, and median survival of 6 to 8 months is

Chemotherapy for advanced gastric and gastroesophageal junction carcinomas remains suboptimal. Both irinotecan (Camptosar) and cisplatin (Platinol) are active against this group of malignancies. This article focuses

This article by Yao, Shimada, and Ajani accurately describes the current state of the art of adjuvant therapy for gastric cancer. The authors’ primary conclusion and current recommendations are as follows:

Yao and colleagues present a concise, yet complete review and analysis of adjuvant therapeutic approaches for gastric adenocarcinoma. They confirm a fact known to all clinical oncologists who manage patients with resected gastric cancer: No adequate data support the value of postoperative (adjuvant) or preoperative (neoadjuvant) therapy in managing patients with locally advanced adenocarcinoma of the stomach.

Gastric carcinoma is a discouraging disease. Although we can clearly identify patient- and tumor-related variables that predict outcome, the only reproducible treatment- related variable associated with an improvement in survival is a complete (R0) resection.[1]

Gastric cancer is often advanced and unresectable at diagnosis. Even when a curative resection is possible, the 5-year survival rate for patients with T2 or higher tumors is less than 50%. Survival rates are even lower if lymph node metastases are present at surgery. Many phase III trials of adjuvant therapy have been conducted around the world during the past 4 decades, but their interpretation varies in the East and West. In the West, postoperative treatment modalities have not proven to be superior to postsurgical observation alone. Thus, at present, the routine use of postoperative therapy should be discouraged. In the Orient, however, routine use of postoperative chemotherapy and/or immunotherapy is common after a surgical procedure. Further investigations that correlate treatment response with molecular markers are needed. Improved clinical trial designs, including better preoperative staging, standardized surgical techniques, inclusion of adequate numbers of patients, and the continued use of a surgery-alone control group, are essential. In addition, the incorporation of newer active agents, radiotherapy, and new strategies, such as preoperative therapy and selection of patients based on tumor biology, would result in much-needed advances. Less toxic approaches with novel mechanisms of action, such as antiangiogenesis therapy, tumor vaccines, monoclonal antibodies, and matrix metalloproteinase inhibitors, also hold promise. [ONCOLOGY 13(11):1485-1494, 1999]

Locally advanced or metastatic adenocarcinoma of the stomach still carries a poor prognosis, with 5-year survival rates of < 15%. Palliative chemotherapeutic regimens for this disease are largely 5-FU–based. We

Although gastric carcinoma is an uncommon disease in North America, its incidence is alarmingly high in Asia, South America, Eastern Europe, and countries of the former Soviet Union. Screening for gastric carcinoma is performed only on a limited basis in Japan; in the rest of the world, therefore, patients often present with advanced disease at the time of diagnosis.

In a laboratory study using an experimental peritoneum model of gastrointestinal cancer, the UFTP [tegafur and uracil (UFT) plus cisplatin] regimen was shown to provide a survival benefit compared with the UFTM (UFT plus