Response and Development of Immune-Related Adverse Events in an 83-Year-Old Man With Metastatic Urothelial Cancer
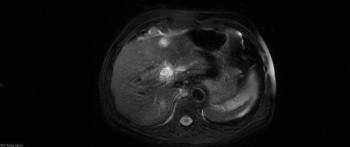
An 83-year-old man was diagnosed with multiple low-grade transitional cell carcinomas over a 6-year period. A surveillance cystoscopy in year 7 showed high-grade noninvasive papillary urothelial carcinoma in the bladder trigone. A CT urogram showed a soft-tissue mass with diffuse enhancement in the lower pole of the left kidney, concerning for malignancy.









































