Genitourinary Cancers
Latest News

Latest Videos

More News
Continued approval for durvalumab in previously treated adult patients with locally advanced or metastatic bladder cancer was dependent upon results from the phase 3 DANUBE trial in the first-line metastatic bladder cancer setting, which did not meet its primary end points in 2020.

The biologics license application is for the locally administered fusion protein Vicineum for the treatment of high-risk, bacillus Calmette-Guérin (BCG)–unresponsive non-muscle invasive bladder cancer.

In patients with cisplatin-ineligible, locally advanced or metastatic urothelial cancer who received prior PD-1/PD-L1 inhibitors, enfortumab vedotin induced the highest response rates seen with any regimen in this setting.
In non-muscle invasive bladder cancer carcinoma in-situ that is not responsive to BCG alone, the addition of N-803 leads to efficacy while maintaining safety.

Adjuvant therapy with nivolumab was superior to placebo for improving disease-free survival in patients with muscle-invasive urothelial carcinoma.

Recently reported data continue to support the use of enfortumab vedotin in patients with advanced urothelial carcinoma who had previously been treated with chemotherapy and immunotherapy.
Using the single-agent atezolizumab (Tecentriq) plus chemotherapy versus chemotherapy alone to treat patients in the front-line setting with cisplatin-ineligible IC2/3 advanced or metastatic urothelial carcinoma (mUC) revealed additional clinical evidence.

Avelumab plus best supportive care as frontline maintenance therapy produced a favorable benefit-risk balance for Japanese patients with advanced urothelial cancer who did not progress on first-line chemotherapy.

High response rates with novel eganelisib plus PD-1 inhibition is reported in patients with metastatic urothelial carcinoma, especially those with low PD-L1 expression.
The procedure provided effective, long-term local control for patients with localized penile cancer, highlighting the need for a multidisciplinary approach to patient care.
The FDA approved pembrolizumab (Keytruda) for the treatment of patients with Bacillus Calmette-Guerin (BCG)-unresponsive, high-risk, non-muscle invasive bladder cancer (NMIBC) with carcinoma in situ (CIS) with or without papillary tumors who are ineligible for or chose to not undergo cystectomy.

Results of this phase III trial could change the current standard of care for treating nonseminomatous or combined germ cell tumors of the testis.
The study found that patients who were randomized to receive avelumab and best supportive care lived significantly longer than those who only received best supportive care.

A new study used two patient-reported measures of sexual function following radical prostatectomy to assess sexual interest and satisfaction in men with prostate cancer.
Nivolumab had limited efficacy against previously untreated brain metastases in patients with metastatic renal cell carcinoma, according to recent trial results.
Researchers compared several glucocorticoid regimens, given in combination with abiraterone acetate, for metastatic castration-resistant prostate cancer.
In this Point/Counterpoint, Drs. Bhat and Punnen argue that active surveillance can be beneficial for patients with intermediate-risk prostate cancer.

In this Point/Counterpoint, Drs. Madueke and Abern argue that active surveillance should not currently be considered for patients with intermediate-risk prostate cancer.
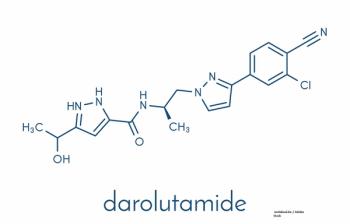
An analysis of the ARAMIS trial looked at quality of life and other outcomes with darolutamide in men with nonmetastatic castration-resistant prostate cancer.

The PAIREDCAP trial looked at different biopsy approaches to see which has the highest cancer detection rate for prostate cancer.
Largest Race-Based Survival Advantage to Date Seen in Men Receiving Sipuleucel-T for Prostate Cancer
A subanalysis of the PROCEED trial showed a surprisingly high survival advantage for a particular race of men receiving immunotherapy for metastatic castration-resistant prostate cancer.
An interim analysis of the CheckMate 920 phase IIIb/IV trial evaluated nivolumab plus ipilimumab in advanced renal cell carcinoma patients with brain metastases.
The KEYNOTE-426 study evaluated pembrolizumab plus axitinib vs sunitinib in patients with metastatic RCC with intermediate/poor risk disease and those with sarcomatoid features.
In this randomized phase III trial, researchers compared cytoreductive nephrectomy vs sunitinib alone in patients with metastatic renal cell carcinoma.
The phase II IMmotion150 study evaluated disease- and treatment-related symptoms in RCC patients receiving atezolizumab alone or in combination with bevacizumab vs sunitinib.