Genitourinary Cancers
Latest News
Latest Videos

CME Content
More News
Experts discuss how data from the CLEAR trial affects the management of advanced renal cell carcinoma in their clinical practices.
Rana McKay, MD, and Tom Powles, MBBS, MRCP, MD, highlight key data from the CLEAR trial and offer their overall opinions on the combination of lenvatinib plus pembrolizumab for the treatment of renal cell carcinoma [RCC].

Arnab Basu, MD, MPH, FACP provides an overview of RCC histologies.
David Aggen, MD, PhD discusses trends in renal cell carcinoma (RCC) incidence.
Oncologists give their initial impressions about the results of a clinical trial studying doublet combination therapies for advanced renal cell carcinoma.
Robert J. Motzer, MD, presents data examined in the article, “Lenvatinib Plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma.”

At the 15th Annual Interdisciplinary Prostate Cancer Congress® and Other Genitourinary Malignancies, Daniel P. Petrylak, MD, spoke about how immunotherapy has influenced treatment for patients with bladder cancer.

Jun Gong, MD, hosted a Twitter takeover during the 2022 Genitourinary Cancers Symposium where he discussed breaking presentations in a #CNRealtimeReport.
Following 2022 ASCO GU, Daniel P. Petrylak, MD; Benjamin H. Lowentritt, MD; Alan H. Bryce, MD; and Tanya Dorff, MD, engage in a rapid-fire question-and-answer 2-Minute Drill program, hosted by CancerNetwork®. Topics include most surprising new data, what needs follow-up, and shameless plug.
Efficacy of adjuvant mitotane for patients with adrenocortical carcinoma following surgery was presented at 2022 ASCO GU.
At 2022 ASCO GU, early trial results show promise for fluorodeoxyglucose and sodium fluoride PET/CT prognostic ability to predict survival in patients with metastatic genitourinary malignancies treated with cabozantinib plus PD-1/CTLA-3 inhibition.

Lead author, Quirin Zangl, MD, spoke with CancerNetwork about research published in the journal ONCOLOGY focusing on the importance comprehensive geriatric assessment tools for patients with genitourinary carcinomas.

This work from Quirin Zangl, MD, and colleagues to evaluate comprehensive geriatric assessment tools to better guide patients with urogenital carcinomas perioperatively and, consequently, to intensify or reduce hospital resource use.

The FDA has granted a full approval to pembrolizumab for the treatment of platinum-ineligible patients with urothelial cancer.
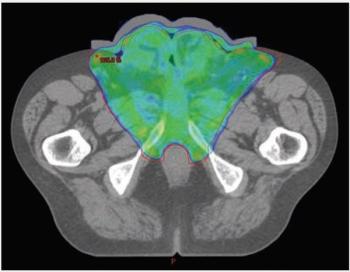
“MK,” a man aged 67 years, presented with fatigue and nausea to his primary care physician. CT staging scans confirmed the primary tumor and a suspicious left 1.2-cm inguinal lymph node but no distant metastases. MRI of the pelvis revealed complete replacement of the penis with tumor as well as invasion into the scrotum and bilateral groin soft tissue; additionally, early pubic bone invasion was present, with left groin lymphadenopathy. Biopsy verified squamous cell carcinoma of the penis, and discussion with the multidisciplinary team uroradiologist confirmed bony invasion.
Robert A. Figlin, MD, details how his institution, Cedars-Sinai Cancer, is aiming to fully understand the cancer journey for those with genitourinary malignancies.

Enfortumab vedotin-ejfv has been approved by the FDA for locally advanced or metastatic urothelial cancer.

Neal D. Shore, MD, FACS, discusses the importance of multidisciplinary care for patients with advanced prostate cancer.

Petros Grivas, MD, PhD, regarding updates in genitourinary oncology that were read out at the meeting.

Petros Grivas, MD, PhD, discusses the impact of avelumab on patients with advanced urothelial carcinoma in the first-line maintenance setting.

Pembrolizumab plus gemcitabine and concurrent hypofractionated radiotherapy to treat patients with MIBC demonstrated safety and efficacy.

Based on results of the recently reported phase 3 CheckMate 274 trial, the FDA has granted priority review designation to nivolumab for the adjuvant treatment of high-risk muscle-invasive bladder cancer.

Accelerated approval for atezolizumab in the treatment of patients with locally advanced or metastatic urothelial carcinoma who are not eligible for certain chemotherapy regimens was maintained by an FDA committee vote.
ONCOLOGY reviews key updates in the treatment of renal cell carcinoma to come out of the Genitourinary Cancers Symposium.

Based on results from the EV-301 and EV-201 trials, 2 separate applications for enfortumab vedotin as therapy for certain patients with previously treated metastatic urothelial carcinoma were accepted by the FDA and granted priority review.