
Achieving undetectable MRD after chemoimmunotherapy predicted longer progression-free and overall survival in CLL patients.

Your AI-Trained Oncology Knowledge Connection!


Achieving undetectable MRD after chemoimmunotherapy predicted longer progression-free and overall survival in CLL patients.

The combination of rituximab and the BCL2 inhibitor venetoclax significantly lengthened PFS among CLL patients in the phase III MURANO trial.

In the DUO and DYNAMO trials, duvelisib improved clinical responses in patients with R/R CLL/SLL and FL, respectively.
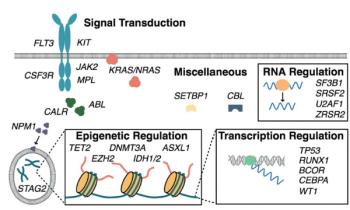
In this first part of our two-part review, we introduce mutation profiling as a relevant clinical tool for hematologists treating patients with myeloid malignancies.

Pooled data show PET imaging of metabolic tumor burden at diagnosis helps identify patients most at risk of FL recurrence.

The FDA has approved brentuximab vedotin (Adcetris) to treat adults with previously untreated stage III or IV cHL, in combination with chemotherapy.

A multicenter team of researchers reports that JAK3/STAT5 mutations are important in ALL and may be targetable.

Three year OS was significantly worse compared with the general population, with a persistent risk for relapse.

Follicular large cleaved cell lymphoma is frequently misclassified, according to researchers.

A large prospective analysis of three large cohorts found a positive link between risk for myeloma and cumulative average young adult and adult BMI.

The US Food and Drug Administration has approved blinatumomab (Blincyto) for patients in remission from B-cell precursor ALL with MRD.

In a phase II study, voxtalisib, which targets all four class I PI3Ks, had efficacy in FL but limited clinical effect in MCL, DLBCL, and CLL/SLL.

In this large population-based, case-control study, having ever used a statin was linked to lower risk of total NHL and certain NHL subtypes, including DLBCL.

BET inhibitors CPI-1205 and CPI-0610 have shown promise in two phase I trials in DLBCL and other lymphomas, investigators at TAT 2018 reported.

Recent FDA approval of front-line brentuximab vedotin with chemotherapy in patients with stage III/IV Hodgkin lymphoma offers the first new treatment for this disease in over 40 years.

A recent phase III study revealed a benefit with pacritinib for patients with myelofibrosis and thrombocytopenia who have experienced treatment failure with previous therapies.

Using CRISPR/Cas9 gene editing to remove CD7 from healthy T cells, researchers have found a way to use third party T cells for CAR-T therapy in T-cell hematologic malignancies.

Researchers have discovered that AML cells require high levels of cholesterol for survival, so the cholesterol pathway could represent a potential therapeutic option for the disease.

In this interview, Dr. Frederick Locke discusses the promise of CAR T-cell therapy for lymphomas, and how this gene therapy could offer hope for patients who don't respond to standard treatments.

Researchers report that PD-1/PD-L1 monoclonal blocking antibody allows T cells to remain active and fight malignant evolution, subsequently preventing tumor resistance.

Researchers examined more than 750 pedigrees of familial hematologic malignancies and found that the same genetic alteration may be responsible for Langerhans cell histiocytosis, acute myeloid leukemia, and primary idiopathic myelofibrosis.

FL progression risk at 2 years was twice as high in the high-risk vs the low-risk group, and the gene-expression score may help to guide treatment.

DLBCL patients who received in-hospital chemotherapy had a lower risk of death during hospitalization.

Nearly 80% of R/R myeloma patients with severe renal impairment requiring hemodialysis achieved disease control with this treatment combination.

The US FDA has granted Priority Review designation for tisagenlecleucel (Kymriah) for treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma who are ineligible for, or have relapsed after, ASCT.