Leukemia
Latest News
Latest Videos

CME Content
More News
Ricardo Parrondo, MD, discusses the potential of combining BTK and BCL2 inhibitors in chronic lymphocytic leukemia treatment, emphasizing their synergistic effect and cautioning against use in frail patients or those with cardiovascular comorbidities, while Pooja Advani, MBBS, MD, stresses the importance of medical history and risk stratification, especially regarding cardiovascular adverse effects.

Fred Hutch and City of Hope "Face Off" regarding presentations focusing on multiple myeloma and chronic myeloid leukemia.

Jay Yang, MD, reviews data from the OPTIC trial, a ponatinib dose-ranging study in chronic-phase chronic myeloid leukemia.

A panel of experts on chronic myeloid leukemia from Karmanos Cancer Institute and Cleveland Clinic introduce themselves and prepare for a team-vs-team debate surrounding recent data in the disease space.

Experts discuss that although first-generation BTK inhibitors such as ibrutinib were groundbreaking for CLL treatment, the field is now shifting toward second-generation options because of their better toxicity profiles. However, the choice between second-generation inhibitors should be personalized, considering individual patient profiles and adverse effects.

Experts highlight that second-generation BTK inhibitors offer improved toxicity profiles, lower cardiovascular risks, and are well tolerated for long-term CLL treatment.

Investigators report that bendamustine/rituximab debulking appears to decrease tumor lysis syndrome risk in patients with previously untreated chronic lymphocytic leukemia.

Data from a phase 1 study support the orphan drug designation for SLS009 as a treatment for patients with acute myeloid leukemia.

Elevated white blood cell counts also appear to correlate with shorter overall survival in patients receiving standard induction chemotherapy plus cytarabine for acute myeloid leukemia.
Patients receiving ibrutinib plus venetoclax for chronic lymphocytic leukemia appear to experience improved overall survival at 46 months compared with those receiving chlorambucil plus obinutuzumab in the phase 3 GLOW study.

SEQUOIA trial data show significant benefits in progression-free survival for patients with chronic lymphocytic leukemia treated with single-agent zanubrutinib, even without a high-risk 17P mutation; Pooja Advani, MD, MBBS, notes that second-generation BTK inhibitors show lower rates of atrial fibrillation and flutter compared with first-generation ones, suggesting increased cardiac safety.
Ricardo D. Parrondo, MD, discusses monotherapy and combination BTKi therapy options for chronic lymphocytic leukemia, noting that the decision depends on patient preferences and disease risk.

Expert insight into the later-line efficacy and tolerability of olverembatinib, a third generation TKI, in ponatinib-resistant, refractory chronic myeloid leukemia populations.

Considerations for selecting between ponatinib and asciminib as third-line therapies for CML based on factors such as disease burden, mutations, and patient tolerability.

Mayo Clinic experts note that targeted therapies for chronic lymphocytic leukemia are generally superior to chemoimmunotherapy, with treatment choices tailored to patient comorbidities and preferences, and noting that real-world data mostly aligns with clinical trials.

In a virtual discussion hosted by Asher A. Chanan-Khan, MD, MBBS, experts from Mayo Clinic discuss treatment strategies for chronic lymphocytic leukemia, including the role of cardio-oncology and various therapeutic approaches.

Results from the phase 3 COG ALL1331 trial found the use of blinatumomab for pediatric patients with B-cell acute lymphoblastic leukemia improved efficacy.

Based on findings from the phase 1/2 BCHILD trial, the FDA approved bosutinib for pediatric chronic myelogenous leukemia.

A review of promising results from the ASCEMBL study, highlighting asciminib's superior efficacy, well-tolerated profile, and potential impact on chronic myeloid leukemia treatment options.

A panel of experts discusses the challenges and considerations surrounding cardiovascular risks associated with ponatinib treatment for chronic myeloid leukemia, emphasizing the need for multidisciplinary collaboration and individualized treatment strategies.

The agent, which is a recombinant Erwinia asparaginase or crisantaspase, can be given to those with acute lymphoblastic leukemia and lymphoblastic lymphoma intravenously and intramuscularly.

Treatment with ibrutinib significantly reduces the risk of initiating next treatment in patients with chronic lymphocytic leukemia or small lymphocytic lymphoma compared with bendamustine/rituximab in a real-world setting.

Experts met to debate recently presented trials in the hematologic oncology after the 2023 American Society of Clinical Oncology Annual Meeting.
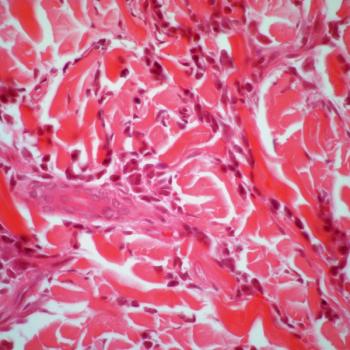
Data from the phase 3 ASCERTAIN trial support the European Commission’s approval of oral decitabine and cedazuridine as a treatment for those with newly diagnosed acute myeloid leukemia.
Several discrepancies in care must be considered to properly address psychological needs in patients with lymphoma, including patient/doctor communication, information provision, and applying distress guidelines.