Multiple Myeloma
Latest News
Latest Videos

CME Content
More News

Treatment with carfilzomib for multiple myeloma was associated with increased incidence of cardiovascular adverse events, especially with higher doses.

Continuous treatment with lenalidomide/dexamethasone significantly improved survival in patients with transplant-ineligible newly diagnosed multiple myeloma.

MMG49 has been identified as a monoclonal antibody that can be targeted using CAR T-cell therapy for patients with multiple myeloma.

An investigational antibody-drug conjugate that targets a key B-cell maturation antigen, offers “deep and durable” responses to some patients with relapsed/refractory multiple myeloma, suggest preliminary findings from the expansion part of the phase I DREAMM-1 trial.

Adding daratumumab to either of two standard-of-care treatment regimens induces rapid, deep, and durable responses and improves progression-free survival in patients with relapsed/refractory multiple myeloma, confirm updated findings from the open-label phase III POLLUX and CASTOR trials.

A single treatment with a second-generation CAR T-cell treatment elicited an overall response rate of 94% in a small study of patients with heavily pretreated multiple myeloma, according to the results of a phase I study presented at the ASH Annual Meeting.

Continuous lenalidomide therapy improves outcomes among patients with newly diagnosed myeloma who are not autologous stem cell transplant candidates, according to findings from a large phase III clinical trial.

The 4-year overall survival rate is 82.3% among multiple myeloma patients eligible for autologous stem cell transplantation, according to a retrospective study.

In this interview we discuss the goals of therapy in multiple myeloma, treatment combinations and transplantation, and how markers such as minimal residual disease are used.

Do you know when to switch treatment combinations or stop certain treatments altogether in the management of patients with multiple myeloma? Take part in a series of case scenarios and see if you can pick the correct course of action for each in our latest quiz.

Elevated pre-transplant C-reactive protein was associated with worse overall survival in patients who underwent autologous stem cell transplantation for multiple myeloma, particularly in those who had transplant more than 12 months after diagnosis.

The BCL-2 inhibitor venetoclax had an acceptable safety profile and showed evidence of activity in patients with relapsed/refractory multiple myeloma.

Are you up to date on the NCCN guidelines for multiple myeloma? And do you know the best way to treat a 66-year-old patient with a lytic rib mass that shows infiltration by kappa light chain restricted plasma cells on core needle biopsy? Test your knowledge in our latest quiz.

Researchers have developed a model that has prognostic value in predicting favorable or unfavorable responses to immunomodulatory derivatives in patients with multiple myeloma.

Are you up to date on the revised international staging for multiple myeloma? How about appropriate consolidation therapy? Test your knowledge on these topics and more in our latest quiz.

An early-phase study of filanesib, the highly selective inhibitor of kinesin spindle protein, showed that the drug had clinical activity and manageable toxicity when combined with prophylactic filgrastim in patients with heavily pretreated multiple myeloma.

The phase III ALCYONE study showed that daratumumab in combination with bortezomib, melphalan, and prednisone is a viable front-line option for myeloma patients who are not candidates for ASCT.

The FDA has released a statement regarding safety concerns that led to a clinical hold on three trials of pembrolizumab in combination with pomalidomide or lenalidomide for patients with multiple myeloma, and the active role of the FDA in protecting patient safety.

How many people this year are expected to be diagnosed with multiple myeloma in the United States? And how does the rate of incidence differ between black patients and white patients, or between men and women? Test your knowledge on multiple myeloma in our latest quiz.

Prolonged use of bortezomib after treatment with combined bortezomib plus dexamethasone did not result in any significant benefit for patients with relapsed/refractory multiple myeloma compared with bortezomib plus dexamethasone alone, but this less dose-intensive approach could be a feasible option for patients who cannot tolerate the standard regimen.

Patients with newly diagnosed multiple myeloma treated with lenalidomide maintenance therapy after undergoing autologous stem-cell transplantation had significantly improved overall survival compared with observation or placebo.

The FDA placed a clinical hold on three trials of the PD-1 inhibitor pembrolizumab in combination with pomalidomide or lenalidomide for patients with multiple myeloma.
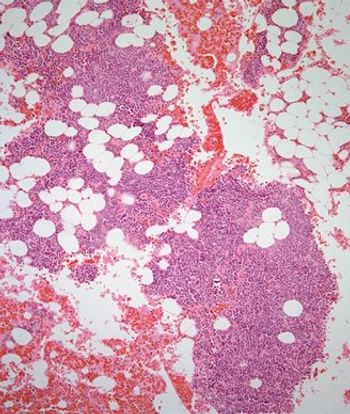
Treatment with the combination of daratumumab plus pomalidomide/dexamethasone resulted in rapid, deep, and sustained responses with no new safety signals in patients with heavily treated multiple myeloma.

Undergoing autologous hematopoietic stem cell transplantation was effective and safe in patients with refractory multiple myeloma, including those patients who are refractory to both proteasome inhibitors and immunomodulatory agents.

The FDA has approved daratumumab in combination with pomalidomide and dexamethasone for the treatment of relapsed or refractory multiple myeloma.