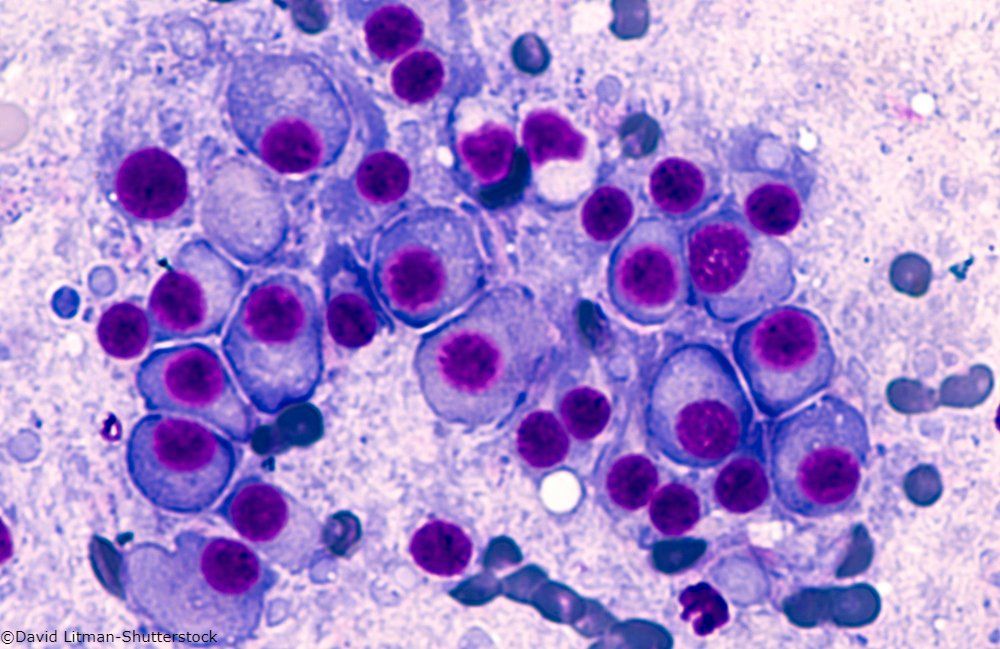
Multiple Myeloma
Latest News
Latest Videos

CME Content
More News

A study shows comprehensive sequencing approach can identify treatment options in patients with relapsed and/or refractory myeloma.

Relapsed or refractory multiple myeloma patients experience high response rates, overall survival with bortezomib-based therapy.

A high level of resilience was associated with a better mental and physical health-related quality of life among patients with multiple myeloma.

Are you up-to-date on treatment regimens for previously treated multiple myeloma? Take our latest quiz to sharpen your knowledge.

From 1990 to 2016, incident cases of multiple myeloma increased by 126% globally, while deaths increased 94%. The US had the most incident cases and deaths.

Investigators concluded ixazomib “may offer a more convenient, active, and well-tolerated alternative to a parenterally administered PI in this setting.”

Our latest quiz highlights the role of bone marrow adiposity in patients with multiple myeloma.

An analysis by FDA and NCI investigators recommended further study of the utility of immunotherapy in patients unable to mount adequate immune responses.

Many MM patients experience a diagnostic interval longer than 3 months until diagnosis is confirmed, and several factors may account for this.

Preoperative pain, prothrombin time activity (PTA), albumin, urine protein, and postoperative chemotherapy appeared to be associated with prognosis.

Our latest quiz highlights the role of testing for minimal residual disease in the management of multiple myeloma.

CAR T-cell therapy with bb2121 induced deep, long-lasting responses in patients with heavily pretreated MM.

In MMY1001, median PFS was 14.1 months and median OS was 21.1 months in patients with lenalidomide-refractory myeloma treated with daratumumab/carfilzomib.

A once-weekly regimen of carfilzomib plus dexamethasone improved response and delayed progression better than a twice-weekly regimen in R/R MM.

OPTIMISMM is the only phase III trial to show a significant PFS benefit in R/R MM patients with prior exposure to lenalidomide.

Therapy with CAR T cells may benefit patients with highly refractory multiple myeloma, said U Penn’s Adam Cohen at ASCO 2018.

Risk stratification is leading to increasing numbers of new molecular targets in multiple myeloma.

A UK team found multiple targets of non-coding mutations, highlighting the importance of broadening the search for cancer drivers into the regulatory genome.

Investigators concluded further investigations are warranted in elderly patients with either stable or responsive disease after bortezomib induction therapy.

Challenge yourself with our latest quiz, covering management considerations in the treatment of patients with transplant-ineligible myeloma.

Take our latest quiz to learn about differences in MM incidence, complications, and treatment among men, women, and various racial and ethnic groups.

Three year OS was significantly worse compared with the general population, with a persistent risk for relapse.

A large prospective analysis of three large cohorts found a positive link between risk for myeloma and cumulative average young adult and adult BMI.

How much do you understand about evaluating prognosis and choosing treatment for patients with myeloma? Take our latest quiz to find out.

The oral therapy combination of ixazomib, pomalidomide, and dexamethasone was effective and well-tolerated in patients with myeloma that had relapsed or was refractory to lenalidomide.