Multiple Myeloma
Latest News
Latest Videos

CME Content
More News

The adverse event profile of single-agent carfilzomib suggests that the agent is an important treatment option for patients with advanced multiple myeloma, particularly those with pre-existing peripheral neuropathy or those who are at risk for the development of peripheral neuropathy.

This article reviews the hematologic safety profile of carfilzomib in patients with relapsed/refractory multiple myeloma, as assessed in a cross-trial safety analysis of four phase II studies, and makes recommendations for the appropriate management of hematologic adverse events.
Patients able to achieve stringent complete response after undergoing autologous stem-cell transplantation for multiple myeloma achieved improved long-term outcomes, including overall survival and time to progression, compared with patients who achieved lesser levels of response.
The results of a univariate analysis have shown that the presence of hyperdiploidy and chromosomal aberrations del(17p13), t(4;14), and +1q21 are all associated with shorter time to progression from smoldering multiple myeloma to active disease.
Researchers have helped to better define the spectrum of light chain (AL) amyloidosis by identifying that AL amyloidosis patients with myeloma who have more than 10% bone marrow plasma cells or those with hypercalcemia, renal failure, anemia, and lytic bone lesions have a similar prognosis to patients with overt myeloma.
Adding vorinostat to bortezomib for the treatment of relapsed multiple myeloma resulted in a statistically significant increase in progression-free survival compared with bortezomib alone, according to results of a new study.

The addition of the oral pan-deacetylase inhibitor panobinostat to bortezomib helped to elicit responses in about one-third of heavily pre-treated patients with multiple myeloma who were refractory of bortezomib, according to the phase II results of the PANORAMA 2 study.

Pomalidomide, an immunomodulatory drug, combined with low-dose dexamethasone improved progression-free survival in patients with refractory or relapsed and refractory multiple myeloma compared with standard of care high-dose dexamethasone, according to a new study.

Numerous small series of patients suggest that the prognosis for non-secretory myeloma patients is likely no worse than the prognosis for patients with traditional secretory myeloma, and in some settings may be superior.

Patients with multiple myeloma who underwent autologous stem cell transplantation may have a continued response to the treatment even after the traditional disease assessment at 100 days. A new study indicates that this continued response maintained prognostic value and should be taken into account when considering post-transplant therapies.

Researchers in London have identified a number of new genetic variants that are linked to myeloma, and one specifically linked to a telomerase RNA component gene called TERC, that helps to control the aging process by acting as a cell’s internal clock.
Early treatment with lenalidomide and dexamethasone in patients with high-risk smoldering myeloma significantly delayed progression to symptomatic disease and prolonged survival with a good safety profile.

Older multiple myeloma patients exposed to novel agents prior to autologous peripheral blood stem cell transplantation were at increased risk for engraftment syndrome.

The International Myeloma Working Group recently released new recommendations to aid physicians in the treatment of bone disease related to multiple myeloma.
Results from a first-in-human trial of daratumumab indicate that the investigational drug reduced paraprotein and bone marrow plasma cells at doses greater than 4 mg/kg in patients with advanced multiple myeloma.

Pomalidomide in combination with low-dose dexamethasone had a highly significant benefit on progression-free survival and overall survival compared with single-agent high-dose dexamethasone in patients with relapsed or refractory multiple myeloma, according to updated results of the MM-03 trial presented at the ASCO 2013 Annual Meeting.

Multiparameter flow cytometry and deep sequencing were both able to accurately identify patients with multiple myeloma who were negative for minimal residual disease, a factor that was found to better predict prolonged survival compared with complete response as measured by traditional response criteria.
Multiple myeloma patients are at increased risk of developing myelodysplastic syndrome or acute leukemia after maintenance lenalidomide or thalidomide treatment, according to a new study.
A majority of patients with multiple myeloma are being treated with novel agents such as thalidomide, bortezomib, and lenalidomide within a year of diagnosis instead of the chemotherapeutic regimens that were more prevalent a decade ago, according to a new study.
An ongoing phase III study comparing the efficacy and safety of perifosine in patients with relapsed or relapsed/refractory multiple myeloma has been discontinued.
African American men with multiple myeloma had a significantly lower frequency of IgH translocations, a signal of nonhyperdiploid multiple myeloma, compared with European American men, according to the results of a new study published in Blood.

The FDA has approved pomalidomide (Pomalyst) to treat patients with relapsed or refractory multiple myeloma who have received at least two prior therapies.

In this interview we discuss the current management and latest treatments and agents in development for multiple myeloma with Dr. Kenneth Anderson of the Dana-Farber Cancer Institute.

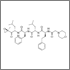
Last week the US Food and Drug Administration approved carfilzomib (Kyprolis) to treat patients with multiple myeloma who have received at least two prior therapies, including treatment with bortezomib (Velcade) and an immunomodulatory therapy.
Three studies published this week show that lenalidomide improves progression-free and overall survival as a maintenance therapy in multiple myeloma, despite its link to other primary cancers.