
A new bioengineered protein that targets two apoptosis receptors produced one dramatic tumor regression and stopped tumor growth in several cases of disease stabilization in 60% of the advanced cancer patients treated in a phase I dose-finding trial

Your AI-Trained Oncology Knowledge Connection!


A new bioengineered protein that targets two apoptosis receptors produced one dramatic tumor regression and stopped tumor growth in several cases of disease stabilization in 60% of the advanced cancer patients treated in a phase I dose-finding trial

the impact the clinical care team can have on the outcome of a health plan's decision to cover a service or treatment

Brain, lung, and ovarian cancers have been chosen as the first cancers to be studied in the pilot phase of The Cancer Genome Atlas (TCGA) project

I read with great interest the paper by Michael Brave and colleagues from the US Food and Drug Administration (FDA). The authors describe the FDA's review supporting this first approval of a chemotherapeutic drug for advanced cervical cancer. This decision was made after a Gynecologic Oncology Group trial (GOG-0179) conducted at 94 American study centers demonstrated a survival benefit in patients with stage IVB, recurrent, or persistent carcinoma of the cervix who received cisplatin plus topotecan (Hycamtin) compared with those who received cisplatin alone.

Topotecan, a camptothecin analog previously approved for the treatment of ovarian cancer and small-cell lung cancer, was granted regular approval by the US Food and Drug Administration (FDA) on June 14, 2006, for use in combination with cisplatin to treat women with stage IVB, recurrent, or persistent carcinoma of the cervix not amenable to curative treatment with surgery and/or radiation therapy. The purpose of this summary is to review the database supporting this approval.

Topotecan, a camptothecin analog previously approved for the treatment of ovarian cancer and small-cell lung cancer, was granted regular approval by the US Food and Drug Administration (FDA) on June 14, 2006, for use in combination with cisplatin to treat women with stage IVB, recurrent, or persistent carcinoma of the cervix not amenable to curative treatment with surgery and/or radiation therapy. The purpose of this summary is to review the database supporting this approval.

Topotecan, a camptothecin analog previously approved for the treatment of ovarian cancer and small-cell lung cancer, was granted regular approval by the US Food and Drug Administration (FDA) on June 14, 2006, for use in combination with cisplatin to treat women with stage IVB, recurrent, or persistent carcinoma of the cervix not amenable to curative treatment with surgery and/or radiation therapy. The purpose of this summary is to review the database supporting this approval.

The US Food and Drug Administration (FDA) has approved the use of a two-drug combination developed by researchers at Rational Therapeutics Institute (Long Beach, Calif) for the treatment of ovarian cancer.

"Although the majority of patients with advanced ovarian cancer will die of the disease, optimism is justified in view of the improved results of surgery followed by cisplatin and carboplatin based chemotherapy."

The FDA has approved Gemzar (gemcitabine HCl for injection, Lilly) in combination with carboplatin for the treatment of women with advanced ovarian cancer that relapsed at least 6 months after initial therapy.

EntreMed, Inc.'s lead drug candidate Panzem (2-methoxyestradiol or 2ME2) has received orphan drug status from the FDA for the treatment of glioblastoma multiforme. In vitro studies in glioma cell lines demonstrated Panzem's antiproliferative activity, and in vivo studies in a preclinical model of glioblastoma showed its antitumor activity, EntreMed said in a press release. Panzem is currently being investigated in a phase II trial in patients with glioblastoma multiforme at the Brain Tumor Center at Duke University Medical Center. The agent previously received orphan drug designation for the treatment of multiple myeloma and ovarian cancer.

A long-term, multicenter study has shown that the reduction in breast and ovarian cancer risk resulting from oophorectomy—the removal of the ovaries and fallopian tubes in women at genetically high risk for these diseases—varies according to the type of genetic mutation present. Specifically, women with mutations in the BRCA1 gene have a greater reduction in ovarian cancer risk following the surgery, while those with BRCA2 mutations have a larger decrease in breast cancer risk. The study results were presented in Atlanta at the American Society of Clinical Oncology annual meeting.

The FDA has approved Hycamtin (topotecan, GlaxoSmithKline) in combination with cisplatin for the treatment of women with late-stage (IVB) cervical cancer that surgery or radiation appears unlikely to cure. The agency acted on the basis of a single phase III trial that showed a significant 2.9-month survival advantage in women treated with the combination vs whose who received cisplatin alone.

Anemia is common in patients treated with chemotherapy for both solid and hematologic malignancies, contributing to fatigue and diminished quality of life and exposing them to the inherent risks of red blood cell transfusions. Erythropoiesis-stimulating proteins have been shown to increase hemoglobin levels, reduce the need for transfusions, and improve quality of life. The current practice guidelines recommend treating moderate to severe chemotherapy-induced anemia with erythropoiesis-stimulating proteins, but the risk of transfusions may be less with earlier intervention at higher hemoglobin levels. A review of the literature suggests that treating mild chemotherapy-induced anemia with erythropoiesis-stimulating proteins reduces the risks of transfusions and the development of more-severe anemia. Weighing the clinical evidence together with other clinical and economic considerations should provide greater insight into the benefits of treating mild anemia in patients treated with chemotherapy.

The FDA has granted Oxigene's combretastatin A4P (CA4P) orphan drug status for treatment of ovarian cancer. In a phase Ib trial, advanced ovarian cancer patients who had failed previous therapy showed a 67% response rate to CA4P plus carboplatin and paclitaxel, the company said in a news release. This combination is currently being evaluated in a phase II trial in women with platinum-resistant ovarian cancer.

A small molecule that inhibits the insulin-like growth factor 1 receptor (IGF-1R) has blocked the development of human colorectal tumors in mice, according to researchers from OSI Pharmaceuticals, Melville, New York.

OXiGENE, Inc, recently announced that the Office of Orphan Products Development of the US Food and Drug Administration (FDA) has granted orphan drug designation to the company's lead vascular-disrupting agent, combretastatin A4 phosphate (CA4P), for the treatment of ovarian cancer.
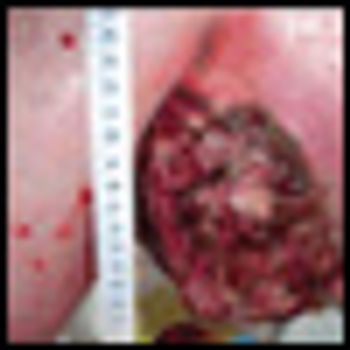
The patient is an otherwise healthy male transferred from an outside hospital with a newly diagnosed melanoma from an unknown primary presenting as a large, left axillary mass.

Rising CA-125 levels are a cause for concern among ovarian cancer

The introduction of newer classes of chemotherapeutic agents, with varying mechanisms of action by which they affect tumor growth and viability, has challenged the traditional norms of clinical trial design and drug approval in oncology. Most notably, the emergence of cytostatic biologic agents with antitumor efficacy has necessitated reassessment of appropriate primary endpoints for phase II and III trials in advanced disease from both a clinical and regulatory standpoint. Recent data in the field establishes an endpoint hierarchy, which places progression-free survival (PFS) between overall survival (OS) and response rate (RR) as appropriate primary endpoints for assessing the clinical efficacy of cytostatic and cytotoxic agents.

Chemotherapy-induced neutropenia (CIN) and its complications exact a substantial toll on patients with cancer. Febrile neutropenia (FN), a sign of life-threatening infections, is associated with lengthy hospitalizations, early mortality, and high medical costs. In addition, neutropenia is the primary cause of dose reductions and dose delays, limiting the delivery of the chemotherapy at full dose and on schedule and thus compromising long-term survival in patients with potentially curable malignancies. Many recent studies in several major tumor types have documented that the greatest risk of neutropenia and its complications is in the first cycle of chemotherapy, with more than 50% of the first episodes of neutropenia and FN occurring in the first cycle. In addition to their other negative effects, these first-cycle events are also associated with early termination of the chemotherapy. The disproportionately high risk of neutropenia in the first cycle has important implications for managing CIN, as well as for the development and use of guidelines for supportive care. It highlights the importance of determining which patients are at high risk for neutropenia and its complications before the chemotherapy is initiated and implementing interventions, such as prophylactic growth factor support in the first and subsequent cycles, to reduce that risk.

As inhibitors of the epidermal growth factor receptor (EGFR) become an increasingly common therapeutic option in cancer, appropriate management of their associated toxicities emerges as a critical part of treatment. Cutaneous manifestations, probably linked to the function of the EGFR in epithelial development, are the most common adverse reactions to EGFR inhibition. The key manifestations are follicular eruptions, nail disorders, xerosis, and desquamation. Growing attention continues to be devoted to the analysis of these events, particularly given their potential role as markers of responsiveness to treatment. However, to date, there are few evidence-based guidelines for the appropriate management of these dermatologic events. Multidisciplinary collaboration between oncologists and dermatologists will be required to improve our understanding and optimize the characterization of these skin toxicities, and to design effective management approaches.

Anti-EGFR (epidermal growth factor receptor) therapies, including tyrosine kinase inhibitors (TKIs) and monoclonal antibodies, demonstrate activity in a variety of tumor types. While both inhibit the EGFR pathway, they act via different mechanisms.

Overexpression of the epidermal growth factor receptor (EGFR) is correlated with poor prognosis in many human cancers. Two main classes of anticancer agents affect the EGFR: those targeting the extracellular ligand-binding domain and those that block the intracellular tyrosine kinase (TK) domain. Cetuximab (Erbitux) is a mouse/human chimeric monoclonal antibody that targets the ligand-binding domain of the EGFR, whereas erlotinib (Tarceva) and gefitinib (Iressa) are small-molecule TK inhibitors. Common toxicities of agents targeting the EGFR differ from those associated with traditional chemotherapy. Given the common pathway through which these agents work, some adverse events are similar. Many patients treated with these agents develop an acne-like rash on the face and upper body, most likely related to keratinocyte alterations and hair follicle proliferation and maturation. Although clinical manifestation of this reaction closely resembles acne vulgaris, the histology is more similar to infectious folliculitis. Other adverse events appear to be related to a drug class or individual agent. For example, interstitial lung disease is a rare but potentially fatal reaction that has been reported with gefitinib. Hypomagnesemia reported in association with cetuximab may be related to EGFR blockade in the kidney. Anaphylactic or anaphylactoid infusion reactions are also seen with cetuximab, as with other monoclonal antibodies.

The FDA's Oncologic Drugs Advisory Committee (ODAC) declined to recommend that the agency approve Gemzar (gemcitabine, Eli Lilly) in combination with carboplatin for the treatment of patients with advanced ovarian cancer that has relapsed at least 6 months after completion of platinum-based therapy.