
Highlight of the Importance of Translational Medicine in Ovarian Cancer

Your AI-Trained Oncology Knowledge Connection!



Highlight of the Importance of Translational Medicine in Ovarian Cancer

In spite of screening recommendations that now begin only at 50 years of age, breast cancer is often diagnosed in women under the age of 40, and there are specific challenges to management of the disease in this younger population.

A superior overall survival with pegylated liposomal doxorubicin (PLD, Doxil) given with trabectedin (T) compared to PLD monotherapy has been demonstrated for advanced, relapsed ovarian cancer.

This article focuses on the recent debate regarding when-or whether-patients with ovarian cancer should undergo aggressive surgical resection.

Whether advanced ovarian cancer should be treated with neoadjuvant chemotherapy or primary debulking surgery is one of the most debated topics in gynecologic oncology.

Surgical debulking of epithelial ovarian carcinoma has been a mainstay of therapy for more than 50 years-since the approach was first advocated by Meigs in 1934.[1] In 1968, Munnell[2] introduced the idea of the "maximum surgical effort”-essentially the removal of as much cancer as possible.

Ginkgo biloba, one of the oldest living tree species, is cultivated worldwide for its medicinal properties and aesthetic appeal. The leaves and seeds are used in traditional medicine to treat respiratory diseases, circulatory disorders, sexual dysfunction, and loss of hearing.
The results of a study that tracked BRCA mutation carriers suggest that women who inherit BRCA gene mutations develop cancer at a younger age than women in the previous generation. The study is published on-line today in the journal Cancer.

The European Society of Gynaecological Oncology (ESGO) biennial meeting is taking place in Milan, Italy, and will run from September 11-14, 2011.

Last week the FDA cleared the use of blood tests for two proteins, HE4 and CA-125; biomarkers that together can be used to estimate risk for ovarian cancer in women who present with a pelvic mass.
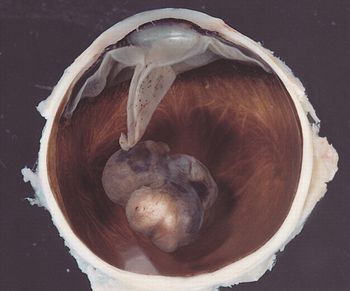
Researchers have discovered that individuals carrying a mutation in the BAP1 gene are at greater risk of developing mesothelioma and uveal melanoma.

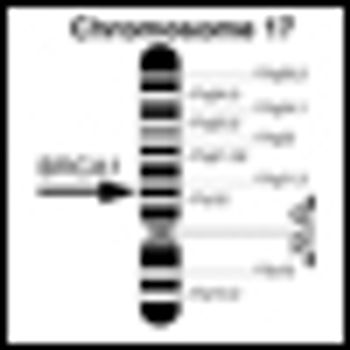
Researchers have identified that “maintenance of global heterochromatin integrity” is a novel function of BRCA1 gene, and propose that this DNA-silencing function is linked to the role of BRCA1 as a tumor suppressor, in an article published in Nature.
A phase II trial of BIBF 1120 (Vargatef) in women with relapsed ovarian cancer that were previously treated with chemotherapy showed that BIBF 1120 given as maintenance therapy resulted in improved progression-free survival rates.
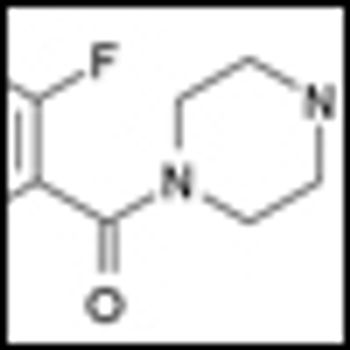
Researchers at the BC Cancer Agency in Vancouver and colleagues have just published the results of a phase II study showing that olaparib (AZD2281), an oral PARP inhibitor, may be effective in treating non-BRCA-related ovarian cancer patients.
Cabozantinib (cabo), formerly known as XL184, has recently shown unprecedented activity against bone metastases in prostate cancer patients in a phase II trial.
Results of a Phase I study of the novel Poly(ADP-ribose) polymerase (PARP) inhibitor veliparib (ABT-888) in solid tumors and lymphoma have just been published in Cancer Research (doi:10.1158/0008-5472.CAN-11-1227).

Many doctors do not properly adhere to current guidelines for offering breast and ovarian cancer counseling and testing services to their female patients, according to a new study from the Division of Cancer Prevention and Control at the CDC.

Scientists at the University of California have taken patient tumor samples and compared them to matched normal tissue samples for two cancer types to map the evolution of mutations in cancer progression.

Two large-scale phase III trials that add bevacizumab to standard chemotherapy regiments in advanced ovarian cancer have reported results at this year's American Society of Clinical Oncology (ASCO).

Live coverage of the ASCO session on PARP inhibitors and DNA repair, with speakers Michael Kastan, Judy Garber, and Elisabeth Plummer.
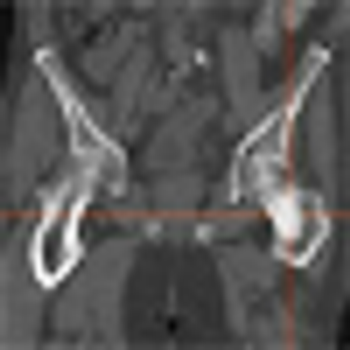
Data from a phase II study of cabozantinib (XL184) in patients with advanced solid tumors show that the drug has activity in both bone and soft tissue. The study evaluated the efficacy and safety of cabozantinib compared to placebo in 9 different solid tumor types including breast, lung, ovarian, and prostate.

A recent study demonstrated that the novel oral Poly(ADP-ribose) polymerase (PARP) inhibitor, olaparib, provided a significant improvement in progression-free survival for women with serous ovarian cancer when used as a maintenance therapy.

In this issue of ONCOLOGY, Golan and Javle present a timely review of the current status of the insulin growth factor-1 receptor (IGF-1R) signaling pathway as a therapeutic target, with a specific focus on gastrointestinal (GI) cancers.

From the current literature

A 40-year-old premenopausal woman with a new diagnosis of invasive lobular carcinoma occurring in a background of lobular carcinoma in situ presents to a multidisciplinary second opinion clinic.