
BELGRADE-Endocyte presented phase IIa data from its trial using EC145 (pegylated liposomal doxorubicin) in women with advanced-stage ovarian cancer at the 2009 European Society of Gynaecological Oncology.

Your AI-Trained Oncology Knowledge Connection!


BELGRADE-Endocyte presented phase IIa data from its trial using EC145 (pegylated liposomal doxorubicin) in women with advanced-stage ovarian cancer at the 2009 European Society of Gynaecological Oncology.

The following company announcements were made at ECCO/ESMO 2009 in Berlin: Pfizer drugs prolong overall survival Pfizer Oncology released data from a phase III trial for sunitinib (Sutent) for the treatment of progressive,

The US Food and Drug Administration (FDA) recently cleared the OVA1 Test, the first blood test that, prior to surgery, can help physicians determine if a woman is at risk for a malignant pelvic mass. OVA1 is the first FDA-cleared laboratory test that can indicate the likelihood of ovarian cancer with high sensitivity prior to biopsy or exploratory surgery, even if radiologic test results fail to indicate malignancy.

Ms. Hydzik's article on intraperitoneal chemotherapy (IPC) for the treatment of ovarian cancer provides the rationale for IPC, presents the supporting evidence, and describes nursing management of these patients through the Memorial Sloan-Kettering Cancer Center experience.

Ovarian cancer is the most deadly gynecologic malignancy. In the US alone, an estimated 21,500 new cases will be diagnosed in 2009, and an estimated 14,600 women will die from this disease.

October marks National Breast Cancer Awareness month, now in its 25th year, a time to contemplate important advances and milestones as well as future research needs.

Exiqon Diagnostics has begun beta testing on the PortiQo Doctor Portal, which will allow oncologists to connect directly to the company.

M.D. Anderson Cancer Center scientists have targeted a protein that is overexpressed in ovarian cancer cells and used it as a molecular homing mechanism to deliver chemotherapy in preclinical models. The protein, EphA2, is attractive for molecularly targeted therapy because its overexpression is associated with poor prognosis, according to Anil K. Sood, MD, and colleagues.

Ovarian malignancies are a leading cause of cancer death in women because they are usually detected in the late stages when the disease is incurable. Encouraging new research presented by Abbott at the American Association for Clinical Chemistry annual meeting,

WASHINGTON, DC-Two separate new drug applications, for the treatment of relapsed ovarian cancer and advanced breast cancer, failed to win the approval of FDA’s Oncologic Drugs Advisory Committee.

Granulosa cell tumors of the testis are very rare neoplasms. While most appear to have a benign course, they occasionally metastasize.
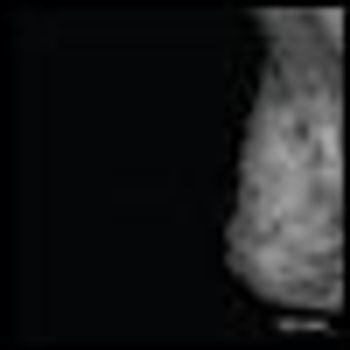
This feature examines the case of a patient with newly diagnosed breast cancer in the setting of a first-trimester pregnancy presenting to our multidisciplinary breast cancer clinic.

Robyn was 63 years old when she was diagnosed with Stage III ovarian cancer. After recovering from a total abdominal hysterectomy and oopherectomy, she traveled to a comprehensive cancer center to consult with a physician specializing in ovarian cancer. She took her entire collection of pathology slides and reports, laboratory and imaging study reports, and the summary of her surgical procedure.

ORLANDO-For the majority of women who undergo ovarian cancer treatment, disease relapse is a matter of when rather than if. These women could spend the rest of their lives undergoing regular CA125 serum marker testing. A recent study that compares the quality of life in early- and advanced-stage ovarian cancer survivors found that CA125 marker measurements for recurrence were, understandably, a source of anxiety for both groups.

Researchers from the Centers for Disease Control and Prevention (CDC) have found that survival among women with ovarian cancer is influenced by age of menarche and total number of lifetime ovulatory cycles.Previous studies have indicated that the factors associated with a decreased risk of developing ovarian cancer include fewer lifetime ovulatory cycles, higher parity, oral contraceptive use, hysterectomy and tubal ligation, according to the researchers.

DENVER-Studies at AACR 2009 demonstrated the early identification of patients who may be susceptible to various forms of leukemia and lymphoma, and hinted at therapies for prevention and treatment.

The National Functional Genomics Center has launched a consortium that will undertake two projects: A biologically-driven analysis of ovarian cancer cell lines and a trial that uses molecular profiling to target chemotherapy.

The American Civil Liberties Union and the Public Patent Foundation at Benjamin N. Cardozo School of Law filed a lawsuit charging that patents on two human genes associated with breast and ovarian cancer stifle research that could lead to cures and limit women’s options regarding their medical care.

Highly expensive imaging technologies are a lightning rod in today’s contentious healthcare landscape. Critics contend that imaging services are grossly overused, while supporters argue that proper use of imaging saves lives and reduces downstream cancer costs.

The right to patent human genes has long been a subject of intense debate. Critics contend that this practice infringes on human privacy and stifles scientific progress. The ACLU has finally got a case it can sink its legal teeth into: a woman who tested positive for gene predisposing her to ovarian cancer was denied access to a second opinion because of current patent law.

Desert living presents many challenges: extreme weather, lack of water, unfriendly cacti, and lethal creatures. Adaptability plus a strong survival instinct are key. David S. Alberts, MD, has plenty of both. When he relocated to the University of Arizona, he’d just finished up five years at the University of California, San Francisco, pouring his efforts into leukemia and myeloma research.

The Centers for Medicare and Medicaid Services has opened a new chapter in the practice of PET with the announcement for a national Medicare payment policy that expands coverage of PET scans in the initial treatment strategy of most solid cancers and for myelo

Prostate-specific antigen testing, the most widely used screening tool in prostate cancer, has long had both critics and supporters. Two studies published in the New England Journal of Medicine continue to generate debate over the value of PSA screening. The papers have two major points in common: They are large-scale studies, and they leave more questions than answers.

Numerous preclinical and clinical studies have demonstrated a role for angiogenesis in the growth and progression of breast cancer. Elevated vascular endothelial growth factor (VEGF) levels have been demonstrated in association with poor outcomes, and thus, this finding is an attractive target for therapeutic intervention.

Ms. D is a 45-year-old woman with ovarian cancer and hepatic metastatic disease. She has received multimodal treatment over the past 5 years. Ms. D lives in her own home, is divorced, and is a single parent of two adolescent children. Her mother is her primary caregiver and also has a deteriorating health condition.