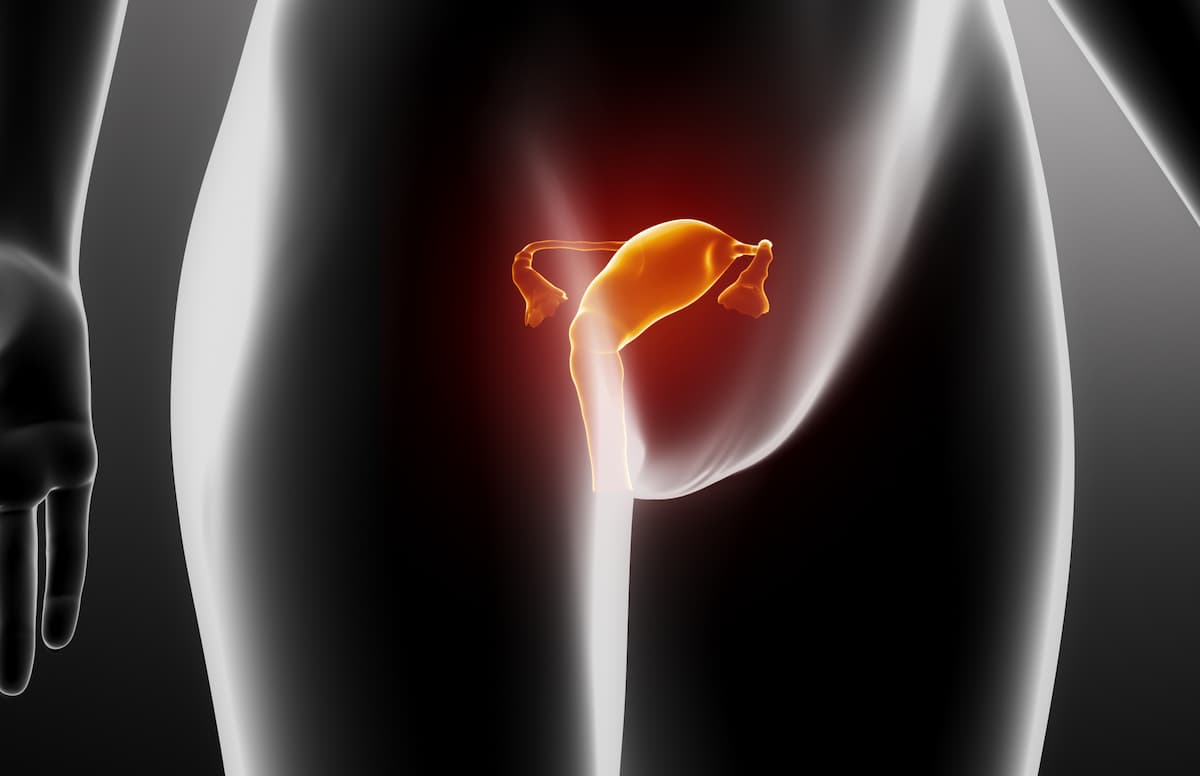
Ovarian Cancer
Latest News
Latest Videos

CME Content
More News

Investigators are assessing avutometinib plus defactinib as a treatment for those with low-grade serous ovarian cancer as part of the phase 3 RAMP 301 trial.
We present the case of a 51-year-old woman with metastatic International FIGO stage IIIC ovarian cancer who had delayed her therapy after initial laparoscopy due to COVID-19 infection and presented with an extreme case of surgical port metastasis.
Patients with advanced ovarian cancer who received primary or interval cytoreduction surgery experienced higher rates of overall and progression-free survival.

The FDA has set a Prescription Drug User Fee Act date of June 29, 2024 for SH-105 as a treatment for patients with ovarian cancer or breast cancer.
Investigators are assessing the safety and antitumor activity of rinatabart sesutecan in advanced epithelial ovarian cancer as part of the phase 1/2 PRO1184-001 study.

Investigators note that the benefit of maintenance olaparib rechallenge in previously treated, platinum-sensitive ovarian cancer extends to the BRCA-mutant and non-mutant cohorts.
The efficacy of HIPEC plus cytoreductive surgery appears to be consistent across ovarian cancer subgroups based on age, histological type, and pre-surgery laparoscopy in the phase 3 OVHIPEC-1 trial.

Implementing exercise programs into standard oncology care may help improve quality of life for patients with ovarian cancer.

Data from the phase 3 MIRASOL trial support mirvetuximab soravtansine as a new standard of care for patients with folate receptor α–positive, platinum-resistant ovarian cancer, says Kathleen N. Moore, MD, MS.

Results from phase 1 of the RAMP 201 study shows efficacy in treating patients with recurrent low-grade serous ovarian cancer with avutometinib plus defactinib, regardless of previous treatment.
Preliminary overall survival data with atezolizumab plus bevacizumab and chemotherapy in recurrent ovarian cancer in the phase 3 ATALANTE trial appear to warrant further analysis.

Collaboration among surgical specialties allows for safe removal of all visible tumors, thus improving survival in patients with advanced ovarian cancer, says Donal J. Brennan, MB, PhD.
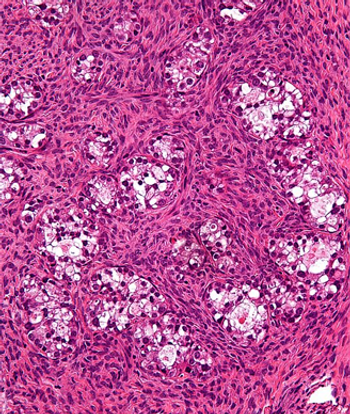
Mirvetuximab soravtansine-gynx may have the potential to become a new standard for patients with folate receptor α–positive, platinum-resistant ovarian cancer, experts say.

Data from the phase 3 MIRASOL trial support the European marketing authorization application for mirvetuximab soravtansine as a treatment for folate receptor α–positive, platinum-resistant ovarian cancer.

Patients with recurrent ovarian cancer did not see an improvement in efficacy when given atezolizumab plus chemotherapy and niraparib.

Results from the phase 3 FLAMES trial show an improved progression-free survival benefit with senaparib monotherapy vs placebo across all pre-specified patient subgroups with newly diagnosed, advanced ovarian cancer.

Investigators of a phase 2 study suggest that relacorilant may add to a ‘sparse’ field of effective treatment options for patients with platinum-resistant or refractory ovarian cancer.

Investigators are assessing AVB-001 as a treatment for those with high-grade serous adenocarcinoma of the ovary, primary peritoneum, or fallopian tube in a phase 1/2 trial.

IDE161 is now eligible for inclusion in a development program allowing for expediated regulatory review following its FDA fast track designation status for advanced BRCA1/2-mutant ovarian cancer.

A planned phase 1 trial will examine CDK12/13 inhibitor CT7439 in patients with several types of solid tumors, including breast and ovarian cancer, as well as Ewing’s sarcoma.
Patients with low-grade serous ovarian cancer appear to have worse survival outcomes following treatment with neoadjuvant chemotherapy.

Findings from an exploratory subgroup analysis in the phase 3 INNOVATE-3 trial suggest that Tumor Treating Fields plus paclitaxel may confer a survival benefit in patients who received only 1 prior line of therapy for platinum-resistant ovarian cancer.

Interim data reveal favorable responses in patients with low-grade serous ovarian cancer treated with avutometinib plus defactinib, according to Susana N. Banerjee, MD.

The safety profile of upifitamab rilsodotin in the phase 1b/2 UPLIFT trial is consistent with prior reports of the agent in those with platinum-resistant ovarian cancer.

Treatment with batiraxcept plus paclitaxel did not produce any new safety signals among patients with platinum-resistant ovarian cancer in the phase 3 AXLerate-OC trial.