Prostate Cancer
Latest News
Latest Videos

CME Content
More News

Anemia in patients who receive talazoparib plus enzalutamide for metastatic castration-resistant prostate cancer appears to be manageable without any compromises in patient-reported outcomes and quality of life.

Findings from the phase 3 PROpel trial suggest that olaparib plus abiraterone acetate may help patients with metastatic castration-resistant prostate cancer live longer, according to Neal Shore, MD, FACS.
Testing for HRR status prior to a diagnosis of metastatic castration-resistant prostate cancer may allow for earlier treatment and potentially greater clinical benefit with olaparib, according to Daniel J. George, MD.
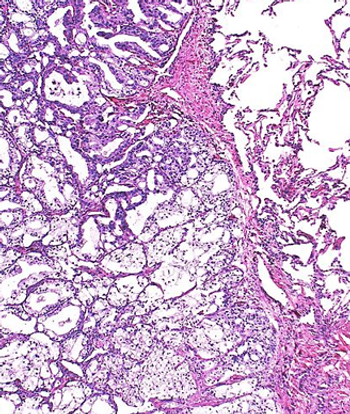
Results from a post hoc sensitivity analysis of the phase 3 ARASENS trial appear to affirm the primary overall survival analysis findings.
Data from the GETUG-AFU 18 trial support high-dose radiation plus long-term androgen deprivation therapy as a standard of care in high-risk prostate cancer.

Four of 5 patients with prostate cancer and ctDNA positivity had disease progression following treatment with darolutamide plus androgen deprivation therapy in a phase 2 trial.

Six months of intensified androgen deprivation therapy plus next-generation anti-androgens may be an attractive option for those with prostate cancer and rising prostate specific antigen levels following prostatectomy.

The overall incidence of bone fractures with prior EBRT to the bone in patients with metastatic castration-resistant prostate cancer remains low.
Additional education on tumor testing and PARP inhibitor therapy is warranted for patients with metastatic castration-resistant prostate cancer, according to Neal Shore, MD, FACS.

Findings support cabozantinib plus atezolizumab as a potential new treatment option for those with metastatic castration-resistant prostate cancer that has progressed on a novel hormonal therapy.

The FDA previously granted approval to frontline olaparib plus abiraterone/prednisone in May 2023 for BRCA1/2–, DDR–altered metastatic castration-resistant prostate cancer.

Darolutamide correlates with a marginally longer length of stay per hospitalization compared with placebo among those with metastatic hormone-sensitive prostate cancer.
While the overall survival between patients receiving cabazitaxel and those receiving Lu-PSMA-617 had negligible difference, the results of the phase 2 TheraP trial supports the use of Lu-PSMA-617 as an alternative treatment for prostate cancer.

Compared with surgery and radiation, focal therapy improves quality of life as well as decreases financial toxicity for patients with prostate cancer.
Including minority populations in clinical trials can be most easily done through outreach and partnerships with other health centers, according to Adam B. Murphy, MD, MBA, MSCI.

Artificial intelligence models may be “seamlessly incorporated” into clinical workflow in the management of prostate cancer, says Eric Li, MD.

Realigning value-based models to increase reimbursement for hypofractionated radiotherapy in prostate cancer may minimize barriers to access among underserved communities.

Robust genetic testing guidelines in the prostate cancer space must be supported by strong clinical research before they can be properly implemented, says William J. Catalona, MD.

Radiographic progression-free survival outcomes with olaparib plus abiraterone acetate and prednisone appear to be consistent with prior analyses of the phase 3 PROpel trial.

Docetaxel plus standard of care may be beneficial among patients with prostate cancer and low prostate-specific antigen levels who do not have any highly effective treatment options, says Anthony D’ Amico, MD, PhD.

Financial constraints and a lack of education among some patients and providers must be addressed to improve the real-world use of certain prostate cancer therapies, says Neeraj Agarwal, MD.

Results from a phase 1 of study of 177Lu-PSMA-617 in combination with pembrolizumab show significant anti-tumor activity with low toxicity in patients with metastatic castration-resistant prostate cancer.

With the announcement that the supply of 177Lu vipivotidete traxetanis now unconstrained, the targeted radioligand will be available in over 200 centers for patients with prostate cancer.

Novel anti-PSMA monoclonal antibody rosopatamab is capable of carrying a bigger payload of radiation particles, which may potentially reduce doses for patients with prostate cancer, says Neeraj Agarwal, MD.
Investigators will assess the safety and preliminary anti-tumor activity of ONCT-534 in metastatic castration-resistant prostate cancer in the phase 1/2 study ONCT-534-101.