Including minority populations in clinical trials can be most easily done through outreach and partnerships with other health centers, according to Adam B. Murphy, MD, MBA, MSCI.

Your AI-Trained Oncology Knowledge Connection!


Including minority populations in clinical trials can be most easily done through outreach and partnerships with other health centers, according to Adam B. Murphy, MD, MBA, MSCI.

Surgery after neoadjuvant immune checkpoint inhibitor therapy for renal cell carcinoma is safe even in challenging surgical cases, according to Jason Scovell, MD.

If approved, UGN-102 may become the first non-surgical option for patients with low-grade intermediate-risk non–muscle invasive bladder cancer, says Sandip Prasad, MD.
Data from the phase 3 CS-003 study underscore the importance of long-term follow-up findings with novel therapies for high-grade non–muscle-invasive bladder cancer, says Stephen A. Boorjian MD.

It is important not to wash over the disparities in treatment access for renal cell carcinoma as merely another statistic and take action on guiding patients to treatment, says Solomon Woldu, MD.

African American and Hispanic patients with clear cell renal cell carcinoma may be less likely to receive treatment with immune checkpoint inhibitors than White patients, says Solomon Woldu, MD.

Patients receiving neoadjuvant chemotherapy plus radical nephroureterectomy for upper tract urothelial carcinoma appear more likely to experience nodal downstaging.
Cretostimogene grenadenorepvec’s efficacy compares favorably with the current nonsurgical standards of care in high-risk, Bacillus Calmette Guerin–unresponsive non-muscle invasive bladder cancer.
Findings from a retrospective analysis indicate that treatment in non-academic cancer centers correlates with a decreased rate of immunotherapy use among patients with advanced clear cell renal cell carcinoma.
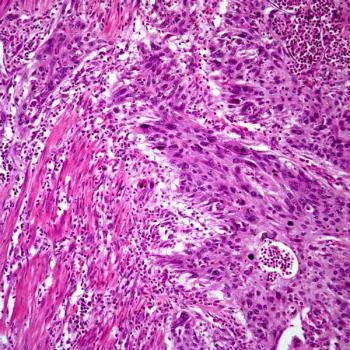
More than half of the patients with non–muscle-invasive bladder cancer in the BOND-003 trial achieve a complete response at 6 months following treatment with cretostimogene grenadenorepvec.

Artificial intelligence models may be “seamlessly incorporated” into clinical workflow in the management of prostate cancer, says Eric Li, MD.

Surgery following treatment with checkpoint inhibitors yields no surgical complications in a cohort of patients with advanced renal cell carcinoma, according to Jason Scovell, MD, PhD.

Robust genetic testing guidelines in the prostate cancer space must be supported by strong clinical research before they can be properly implemented, says William J. Catalona, MD.

An expert from the University of Texas MD Anderson Cancer Center discussed kidney cancer treatment updates from a recent medical conference.

During the 2022 Society for Urologic Oncology (SUO) Annual Meeting, an expert from the Baylor College of Medicine, discussed how perceptions around the use of testosterone therapy have evolved for patients with metastatic prostate cancer.

Thus far, findings from 2 trials show that treatment with bipolar androgen therapy is associated with several benefits in patients with castration-resistant prostate cancer, according to an expert from Sidney Kimmel Comprehensive Cancer Center.

Investigators have observed that treatment with bipolar androgen therapy has suppressed at least one oncogene in patients with castration-resistant prostate cancer who derived a response to therapy.

Future research must address ways to improve the prediction of kidney cancer recurrence to better inform patients, says an expert from the Royal Free London NHS Foundation Trust.

Despite the observed disease-free survival benefit associated with pembrolizumab in high-risk kidney cancer after surgery, the European Association of Urology guidelines maintain a weak recommendation for its use.

Patients with kidney cancer should be made aware of trials that have not read out positively in the adjuvant setting, according to an expert from the Royal Free London NHS Foundation Trust.

Ongoing clinical trials are exploring the cytoreductive properties and immunogenic potential of stereotactic radiation in the treatment of patients with metastatic kidney cancer, according to an expert from the University of Texas Southwestern Medical Center.

“Much more research” is needed to advance the adjuvant treatment of patients with kidney cancer, according to an expert from the Royal Free London NHS Foundation Trust.
Long-term follow-up showed that the use of pembrolizumab monotherapy maintained durable complete responses, while rates of upstaging at the time of radical cystectomy were consistent with previous findings in the KEYNOTE-057 trial in patients with a bladder cancer subset.
At a median follow-up of 17.4 months, half of the enrolled patients with metastatic castration-resistant prostate cancer experienced a decrease in PSA level from baseline following treatment with tazemetostat in combination with abiraterone acetate or enzalutamide.
Findings from an interim analysis of data show that the addition of blue light cystoscopy to conventional white light significantly improved clinical outcomes in a group of patients with non–muscle-invasive bladder cancer.

All patients with chemo-naïve metastatic castration-resistant prostate cancer experienced PSA decline, and half the cohort experienced a PSA decline greater than 50% after treatment with a novel PSMA-targeted radionuclide therapy.

Patients with newly diagnosed prostate cancer benefitted from 18F-rHPSMA-7.3 PET when used prior to surgery.
Patients with cisplatin-ineligible muscle-invasive bladder cancer experienced a better rate of downstaging and improved survival benefit with neoadjuvant pembrolizumab prior to radical cystectomy vs immediate radical cystectomy.

“With more potent agents, can we actually cure patients [with prostate cancer]? I'm not so sure about that…because of heterogeneity,” said an expert from NewYork-Presbyterian – Weill Cornell Medical Center in New York City. “So we'll see. But I think with combinations, maybe someday we're going to be able to get there.”
Theranostics may fill an unmet need for a group of patients with high-risk localized prostate cancer, but more research is needed to identify how oncology providers can integrate it with the standard treatments, according to an expert from the University of Texas MD Anderson Cancer Center.