
Findings from the phase 1/2 MajesTEC-1 study support the European Commission’s approval of teclistamab at a reduced dose frequency for patients with relapsed/refractory multiple myeloma.

Your AI-Trained Oncology Knowledge Connection!


Findings from the phase 1/2 MajesTEC-1 study support the European Commission’s approval of teclistamab at a reduced dose frequency for patients with relapsed/refractory multiple myeloma.
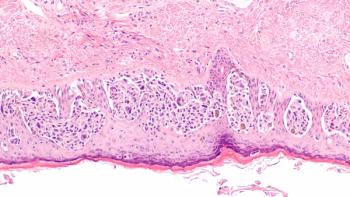
Data from the phase 3 CheckMate-76k trial support the European Commission’s approval of nivolumab as an adjuvant treatment for patients with resected stage IIB or IIC melanoma.
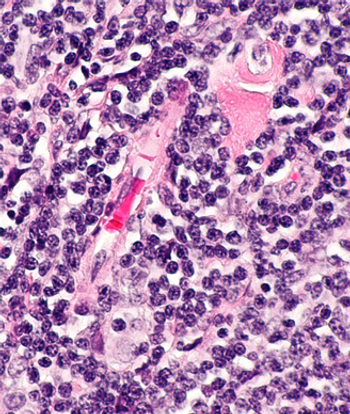
Data from the phase 1 ELM-1 trial and phase 2 ELM-2 trial support the marketing authorization application for odronextamab in relapsed/refractory follicular lymphoma and diffuse large B-cell lymphoma.
The approval from the European Commission is based on findings from the phase 3 TROPiCS-02 study.

JZP458 is part of a multi-agent chemotherapy regimen for lymphoblastic lymphoma and acute lymphoblastic leukemia, and can be used for adult and pediatric patients.

The European Commission will evaluate elacestrant in locally advanced or metastatic breast cancer after receiving a positive opinion on approval from the CHMP.

Findings from the phase 3 KEYNOTE-811 trial support the CHMP’s recommendation to approve pembrolizumab plus trastuzumab in HER2-positive advanced gastric or gastroesophageal junction adenocarcinoma.

Results from the phase 1/2 NP30179 trial led the European Commission to grant conditional marketing authorization to patients with relapsed/refractory diffuse large B-cell lymphoma.

Data from the phase 2 ROCKstar trial support the approval of belumosudil as a treatment for patients with chronic graft-versus-host-disease in Scotland.

Approval of the Droplex EGFR Mutation Test v2 may improve access to appropriate targeted therapies for patients with EGFR-mutated non–small cell lung cancer in Korea.

Data from the phase 3 CheckMate 816 trial support the European Medicines Agency’s Committee for Medicinal Products for Human Use recommendation to approve nivolumab plus platinum-based chemotherapy as a treatment for resectable non–small cell lung cancer.

Supporting data for the supplemental new drug application for toripalimab plus chemotherapy in recurrent metastatic triple-negative breast cancer come from the phase 3 TORCHLIGHT study.
China’s National Medical Products Administration has also approved zanubrutinib as a treatment for patients with Waldenström macroglobulinemia based on findings from the phase 3 ASPEN trial.
Patients in Canada who have advanced non–small cell lung cancer can now receive cemiplimab plus chemotherapy as a first-line treatment following Health Canada’s approval of the regimen.
The European Medicines Agency validates the potential indication of dostarlimab/chemotherapy in mismatch repair deficient/microsatellite instability–high endometrial cancer based on the phase 3 RUBY trial.
Data from the phase 3 MAGNITUDE study support the European Commission’s approval of niraparib plus abiraterone acetate dual action tablets in BRCA-mutated metastatic castration-resistant prostate cancer.
Data from the phase 3 KarMMA-3 trial support several applications for idecabtagene vicleucel in earlier use for triple-class exposed multiple myeloma in the United States, Europe, and Japan.
Findings from 2 phase 3 trials support the recommendation to approve sodium thiosulfate to reduce the risk of cisplatin-related hearing loss in pediatric patients with solid tumors in Europe.

Findings from the phase 3 TRANSFORM study support the Committee for Medicinal Products for Human Use’s recommendation to approve lisocabtagene maraleucel for large B-cell lymphoma.

Findings from 2 clinical trials support the approval of acalabrutinib as a treatment for patients with mantle cell lymphoma in China.

Investigators plan to submit a communication application to seek marketing approval in China for ARX788 as therapy for HER2-positive advanced or metastatic breast cancer.

Disparities in pediatric cancer survival in Europe might be mitigated through cross-border care, twinning programs, and use of pan-Europe cancer control plans.

The phase 3 TORCHLIGHT study, examining toripalimab and paclitaxel, has become the first study in China to achieve positive outcomes with immunotherapy for triple-negative breast cancer.
Adult patients with previously treated unresectable or metastatic HER2-positive breast cancer in China can now receive treatment with trastuzumab deruxtecan.

Patients from China with unresectable or metastatic gastric or gastroesophageal junction adenocarcinoma can now be treated with tislelizumab plus chemotherapy following its approval.
Adult patients with chronic lymphocytic leukemia in the European Union can now receive treatment with acalabrutinib tablets.

Data from the European phase 1 MB-105 trial indicate that the safety profile of annamycin in geriatric advance acute myeloid leukemia are consistent with previously reported findings.

The European Commission approval for trastuzumab deruxtecan in HER2-low metastatic breast cancer is based on data from the phase 3 DESTINY-Breast04 trial.

The National Medical Products Administration of China gives approval to mobocertinib for patients with non–small cell lung cancer harboring EGFR exon 20 insertion mutations.

Japanese patients with relapsed/refractory large B-cell lymphoma can now receive treatment with axicabtagene ciloleucel following its approval.