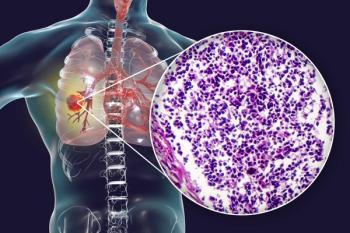
A real-world, retrospective analysis showed that CRS and ICANS occurred in 48% and 16% of patients with ES-SCLC who were treated with tarlatamab.

Your AI-Trained Oncology Knowledge Connection!


A real-world, retrospective analysis showed that CRS and ICANS occurred in 48% and 16% of patients with ES-SCLC who were treated with tarlatamab.

In patients with LS-SCLC who were ineligible for a prophylactic cranial irradiation, toripalimab appeared to decrease the progression of brain metastases.

Cisplatin/etoposide and carboplatin/etoposide achieved median OS of 8.8 months and 7.8 months, respectively, in those with extensive-stage small cell lung cancer.
Data from the IDeate-Lung01 trial support the potential role that ifinatamab deruxtecan may play in the management of extensive-stage small cell lung cancer.

Lurbinectedin achieved an ORR of 27% in all patients with extensive-stage small cell lung cancer in the phase 4 Jazz Emerge 402 study.

Ateganosine plus cemiplimab was well tolerated in patients with heavily pretreated advanced NSCLC, with most adverse effects grades 1/2 in severity.

Findings from the 2025 World Conference on Lung Cancer reflected key updates in the management of NSCLC, SCLC, and other lung cancer types.

All efficacy-evaluable patients with ES-SCLC treated with surufatinib, durvalumab, etoposide, and chemotherapy responded to treatment.

No new safety signals were identified with subcutaneous amivantamab in EGFR-mutant NSCLC, and infusion reactions were reduced vs the IV formulation.

After failing to record any objective responses in 9 patients with relapsed/refractory ES-SCLC, the phase 2 trial was terminated early.

Results from the DeLLphi-303 trial showed sustained efficacy and safety with tarlatamab plus anti–PD-1 treatment for patients with extensive-stage SCLC.

The confirmed overall response rate with ABB-706 was 77% in patients with relapsed/refractory small cell lung cancer who received 2 prior lines of therapy.

When compared with observation, adjuvant crizotinib did not improve disease-free survival or overall survival in ALK-positive NSCLC.

Adding aumolertinib to chemotherapy in the treatment of patients with EGFR-mutated NSCLC led to improvements in progression-free survival.

The median CNS PFS with Dato-DXd was 5.0 months vs 3.0 months with docetaxel in patients with NSCLC who have brain metastases.

For patients with unresectable stage III NSCLC, concurrent durvalumab with chemotherapy/radiation did not show an efficacy improvement.
The safety and tolerability of nivolumab/chemotherapy in non–small cell lung cancer were manageable and consistent with its profiles in other clinical scenarios.

Zidesamtinib was well tolerated in patients who received prior ROS1 TKI therapy with advanced NSCLC, and dose discontinuation/reduction rates were low.

Ivonescimab plus chemotherapy following progression after a third-generation TKI showed consistent efficacy across EGFR-mutated NSCLC subgroups.

Subgroup data from KEYNOTE-671 support the use of perioperative pembrolizumab in stage II or III non–small cell lung cancer of any clinical nodal status.

AE-related discontinuations of osimertinib were low, and no new treatment-related deaths were reported with the combination in EGFR-mutant NSCLC.
Updated findings from the AEGEAN trial support perioperative durvalumab as a new therapy option for those with resectable non–small cell lung cancer.

Phase 2 data show meaningful efficacy with taletrectinib regardless of whether patients with ROS1-positive NSCLC previously received tyrosine kinase inhibitors.

Confirmed partial responses with firmonertinib occurred across a range of EGFR PACC mutations among patients with NSCLC in the phase 1b FURTHER trial.

Patients with EGFR-mutated NSCLC had sustained HRQOL when treated with amivantamab plus lazertinib vs osimertinib.

Data from the HARMONi-2 trial support the potential superiority of frontline ivonescimab vs pembrolizumab in non–small cell lung cancer.

In particular, dato-DXd plus durvalumab and chemotherapy produced the highest pCR and mPR rates in the phase 2 NeoCOAST-2 trial.

Phase 1b data also show encouraging preliminary intracranial activity with zongertinib among patients with HER2-mutant non–small cell lung cancer.

BAY 2927088 showed substantial response rates for patients with pretreated HER2-mutant non–small cell lung cancer.

Amivantamab/lazertinib also reduces the risk of second progression or death compared with osimertinib in the phase 3 MARIPOSA trial.