
Asciminib does not appear to interfere with the general life activities of patients with resistant/intolerant chronic phase chronic myeloid leukemia in the phase 3 ASCEMBL trial.

Your AI-Trained Oncology Knowledge Connection!


Asciminib does not appear to interfere with the general life activities of patients with resistant/intolerant chronic phase chronic myeloid leukemia in the phase 3 ASCEMBL trial.
The FDA also accepts a premarket approval application for the Lumicell Direct Visualization System in the breast cancer space.

Hospitalization rates appear to be comparable between patients receiving trastuzumab deruxtecan and trastuzumab emtansine for HER2-positive breast cancer in the phase 3 DESTINY-Breast03 study.
Investigators say that they do not have any safety concerns regarding GEN-001 and avelumab in patients with advanced gastric or gastroesophageal junction adenocarcinoma.

Adult patients with indolent systemic mastocytosis can now receive avapritinib following the FDA’s approval of the agent.
Findings from a retrospective analysis highlight no significant differences in metastasis-free survival in patients with muscle-invasive bladder cancer who received radical cystectomy vs trimodality therapy.
C. Ola Landgren, MD, PhD, discusses the benefits obtained when patients have access to a full team of diverse caregivers rather than just a single physician.
Santosh Rao, MD, discusses ongoing and potential future research efforts aiming to maximize the benefits that patients with kidney cancer may derive from integrative oncology treatment techniques.
C. Ola Landgren, MD, PhD, reviewed the plethora of emerging treatment options in multiple myeloma, and how unmet needs in the space can be overcome.

Ashley E. Rosko, MD, reviews unmet needs and treatment sequencing for patients with multiple myeloma.
Ben Kong, PharmD, BCPS, shares a perspective on a study of biomarkers published recently in ONCOLOGY.

Santosh Rao, MD, discusses guideline recommendations and therapeutic techniques that underscore the current integrative oncology landscape for patients with kidney cancer.

Patients with relapsed/refractory diffuse large B-cell lymphoma can now receive epcoritamab following the FDA’s approval of the agent.

Trial findings indicate melatonin may be a potentially effective solution for reducing the incidence and severity of cancer-related fatigue among patients with breast cancer.

Patients with human immunodeficiency virus and cancer may derive clinical benefit from immune checkpoint inhibition with no excess toxicity in non–small cell lung cancer and other cancers.
A post hoc analysis indicates continuous intravenous infusion of doxorubicin does not yield additional benefit for patients with soft-tissue sarcoma compared with bolus delivery methods.

Findings from the phase 2 MAJIC-PV trial highlight a superior event-free survival and overall survival following ruxolitinib vs best available treatment in patients with polycythemia vera who were intolerant/resistant to hydroxycarbamide.

Rituximab plus bendamustine and cytarabine continues to show clinical efficacy and favorable outcomes in a population of elderly patients with mantle cell lymphoma.

A phase 2 trial indicates that limited surgery and post-operative proton therapy result in a high rate of tumor control and less severe complications in young patients with craniopharyngioma.
The addition of cabozantinib to nivolumab and ipilimumab also appears to improve responses in patients with advanced renal cell carcinoma in the phase 3 COSMIC-313 trial.

Investigators indicate that more analyses are necessary to identify patients with pretreated small cell lung cancer who will best benefit from treatment with olaparib and durvalumab.

Arshiya Mariam, BS, and colleagues report the findings of a large meta-analysis assessing the ability of various biomarkers to predict responses to immune checkpoint inhibition.

Safety findings from the phase 3 FLAURA2 trial appear to be consistent with the known profiles of osimertinib and chemotherapy for the treatment of those with EGFR-mutated non–small cell lung cancer.

Investigators identify several potential first time risk factors of long-term outcomes in patients with HER2-positive breast cancer with or without a pathologic complete response.

Investigators are expected to share initial safety and efficacy data from a phase 1/2 trial assessing IMPT-314 as a treatment for aggressive B-cell lymphoma in the second half of 2023.

Prexasertib is currently under investigation as part of a phase 2 trial as a treatment for patients with platinum-resistant ovarian cancer, endometrial adenocarcinoma, and urothelial cancers.

The FDA requests additional information requiring time and resources extending beyond the current evaluation period for vic-trastuzumab duocarmazine in advanced HER2-positive breast cancer.
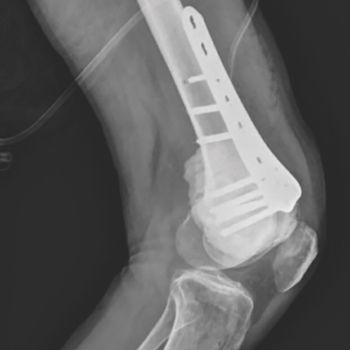
Hans Kristian Nugraha, MD, SpOT, and colleagues detail the potential benefits of an arthroscopic surgical approach through a case study of a patient with giant cell tumor of bone.

Treatment with 20 mg/m2 of lenalidomide in patients under 22 years old who have pilocytic astrocytomas and optic pathway gliomas appears to be safe and effective.

Among those with multiple myeloma, Hispanic patients may experience worse outcomes such as in-hospital mortality vs other ethnic groups.