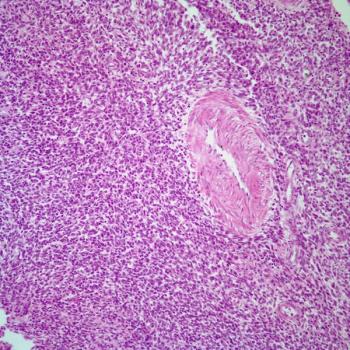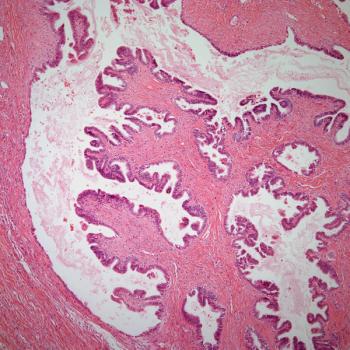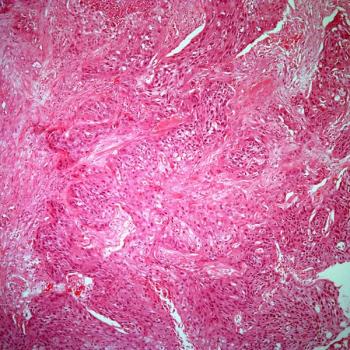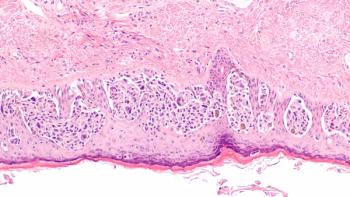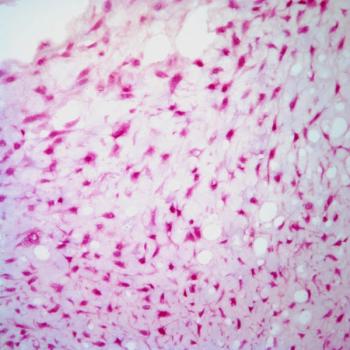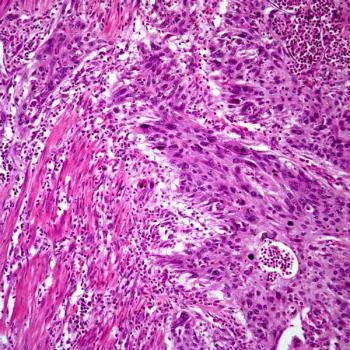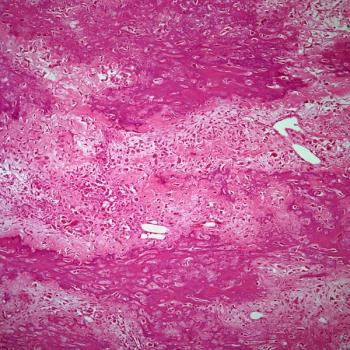
Treatment with lisocabtagene maraleucel correlates with a reduction in CD19-positive cells in responders and patients with stable disease among those with relapsed or refractory chronic lymphocytic leukemia or small lymphocytic lymphoma in the phase 1/2 TRANSCEND CLL 004 trial.