
For clinicians practicing in the community, constant communication and education from those in institutions may help to produce the best quality of care for patients with multiple myeloma.

Your AI-Trained Oncology Knowledge Connection!


For clinicians practicing in the community, constant communication and education from those in institutions may help to produce the best quality of care for patients with multiple myeloma.

18F-rhPSMA-7.3 injection is now available to help identify PSMA-positive lesions during PET imaging for patients with metastatic or recurrent prostate cancer.
Data from the phase 3 DUO-E trial indicate that the safety of durvalumab/chemotherapy with or without olaparib/durvalumab or durvalumab monotherapy maintenance in recurrent endometrial cancer was consistent with previous reports of each agent.
Ropeginterferon alfa-2b-njft has been moved to preferred status in the National Comprehensive Cancer Network guidelines for polycythemia vera based on data supporting the agent’s superior efficacy and safety in high- and low-risk populations.
Findings from the phase 3 DAWNA-2 trial suggest that dalpiciclib plus endocrine therapy may be a potential novel first-line treatment option for hormone receptor–positive, HER2-negative advanced breast cancer.
Patients under the age of 3 with neuroblastoma experienced no significant negative impact on survival when their disease was reclassified from high-risk to intermediate-risk and their therapy was thusly reduced.

Synchronous disease appears to have a more hormone dependent transcriptional profile than metachronous disease, according to a retrospective review of patients with metastatic castration-sensitive prostate cancer.

Ashley E. Rosko, MD, specializes in multidisciplinary care for elderly patients with multiple myeloma, and how to make treatment most accessible to them.

At first relapse, novel therapies are offered to patients with multiple myeloma at The Ohio State University Comprehensive Cancer Center-The James.
Axicabtagene ciloleucel may prolong survival and improve outcomes vs standard-of-care therapy in older patients with relapsed/refractory large B-cell lymphoma.

Ashley E. Rosko, MD, highlights potential changes on the horizon to the standard of care in multiple myeloma therapy, and discussed the personalization of treatment based on transplant eligibility.
Data from the phase 3 FRESCO and FRESCO-2 trials support the new drug application for fruquintinib as a treatment for patients with previously treated metastatic colorectal cancer.
Luspatercept may lead to a paradigm shift in the treatment of patients with lower-risk myelodysplastic syndrome, according to an expert from The University of Texas MD Anderson Cancer Center.
Medicaid expansion may help overcome inequities in access to care for pancreatic and gastric cancers, with notable benefit in Black patients, according to an expert from The University of Texas MD Anderson Cancer Center.

A positive R0 resection rate helped to confirm the noninferiority of minimally invasive distal pancreatectomy compared with open distal pancreatectomy in those with resectable pancreatic cancer.

18F-Fluorestradiol is currently the only imaging agent approved by the FDA for assessing estrogen receptor–positive lesion status to better guide treatment decision-making.

Data from a phase 3 study indicate that weight loss intervention with a telephone-based program appears to be effective across all subgroups of breast cancer survivors.
Pembrolizumab plus chemotherapy with or without bevacizumab yields a survival benefit in PD-L1 positive patients with cervical cancer in the phase 3 KEYNOTE-826 trial.
Sustained improvements in leukemia outcomes in low- and middle-income countries are feasible with cross border programs, according to an expert from the University of California, San Diego.
Voruciclib plus venetoclax appears to yield no dose-limiting toxicities in a small population of patients with acute myeloid leukemia, according to early findings from a phase 1 study.
C. Ola Landgren, MD, PhD, illustrated how minimal residual disease tracking may allow greater treatment personalization in the future.
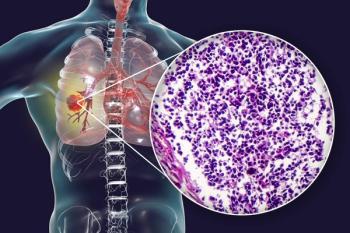
Patients with nonsquamous non–small cell lung cancer experiencing clinical benefit with sitravatinib plus nivolumab in the phase 3 SAPPHIRE trial are eligible to remain on treatment.

Findings from a study suggest that anti-frameshift peptide antibodies may also predict incidence of immune-related adverse effects in patients with lung cancer following immune checkpoint inhibitor therapy.
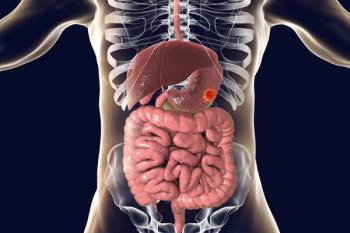
Claudin 18.2–targeting antibody-drug conjugate ATG-022 is under assessment as part of the phase 1 CLINCH study in patients with advanced or metastatic solid malignancies.
Supporting data for the supplemental new drug application for toripalimab plus chemotherapy in recurrent metastatic triple-negative breast cancer come from the phase 3 TORCHLIGHT study.

Manufacturers plan to halt further development of milademetan for liposarcoma following the publication of topline results from the phase 3 MANTRA trial.
C. Ola Landgren, MD, PhD, speaks about transplant eligibility, as well as new therapies and minimal residual disease negativity, in the multiple myeloma space.
Investigators plan to initiate a phase 2 study evaluating AFM13 plus AB-101 in patients with relapsed/refractory classical Hodgkin lymphoma in the first half of 2024.

Plans have been made to file a new drug application in China for roxadustat as a treatment for chemotherapy-induced anemia in those with non-myeloid malignancies.
Mitazalimab is currently under investigation in combination with chemotherapy as a treatment for patients with metastatic pancreatic ductal adenocarcinoma in the phase 2 OPTIMIZE-1 trial.