
According to an expert from University Hospitals, integrative oncology has a place in the treatment of patients with kidney cancer alongside palliative care, psycho-oncology, and physical therapy.

Your AI-Trained Oncology Knowledge Connection!


According to an expert from University Hospitals, integrative oncology has a place in the treatment of patients with kidney cancer alongside palliative care, psycho-oncology, and physical therapy.

Investigators say that among those with platinum-refractory recurrent or metastatic head and neck squamous cell carcinoma who responded to nivolumab plus ipilimumab duration of response was not reached.

An artificial intelligence-based model for detecting lymph node metastases appears to identify tumor micrometastases in bladder cancer that pathologists may miss while classifying patient results as negative.
Combination treatment with IMX-110 and tislelizumab yields no dose-limiting toxicities thus far in the first cohort of patients with advanced/metastatic colorectal cancer in the phase 1b/2a IMMINENT-01 trial.

Common symptoms following debulking surgery for gynecologic cancer appear to include pain, diarrhea, and nausea, according to an expert from Memorial Sloan Kettering Cancer Center.

Pharmacy director Kirollos Hanna, PharmD, BCPS, BCOP, FACC, discusses how to navigate shared toxicities between combination immunotherapy and VEGF inhibitors for patients with renal cell carcinoma.

The FDA’s approval of the FoundationOne Liquid CDx assay may improve access to treatment with mobocertinib for patients with non–small cell lung cancer harboring EGFR exon 20 insertion mutations.

Based on findings from the phase 3 MIRASOL trial, investigators plan to submit a supplemental biologics license application for mirvetuximab soravtansine in folate receptor α–positive platinum-resistant ovarian cancer.

According to an expert from University Hospitals, oncologists should work together and look for opportunities to improve patients’ diets and exercise routines to mitigate symptoms of kidney cancer and associated treatment.
According to the investigators of the INSITE trial, the potential benefit of pegulicianine fluorescence–guided surgery in breast cancer warrants investigation in future studies.
ERAS-801, which now has FDA fast track designation for glioblastoma with EGFR alterations, is currently under investigation as a monotherapy in the phase 1 THUNDERBBOLT-1 trial.
Patients in Canada who have advanced non–small cell lung cancer can now receive cemiplimab plus chemotherapy as a first-line treatment following Health Canada’s approval of the regimen.
THIO plus cemiplimab does not appear to yield any dose-limiting toxicities or significant treatment-related adverse effects in patients with advanced non–small cell lung cancer.
Enzalutamide with or without leuprolide also reduces the risk of prostate-specific antigen progression in those with non-metastatic hormone-sensitive prostate cancer in the phase 3 EMBARK trial.

Liso-cel produces no new safety signals in relapsed or refractory mantle cell lymphoma and follicular lymphoma in the phase 1 TRANSCEND NHL 001 trial and the phase 2 TRANSCEND FL trial.
Data from the phase 3 COMMANDS trial support a supplemental biologics license application for an expanded indication for luspatercept-aamt for lower-risk myelodysplastic syndrome with anemia.
During the ODAC meeting, members voted to restrict the use of olaparib plus abiraterone and prednisone or prednisolone to patients with BRCA-mutated metastatic castration-resistant prostate cancer.

xT CDx is a 648-gene next-generation sequencing panel capable of determining microsatellite instability status in patients with colorectal cancer.

Javier Orozco-Mera, MD, FACS, MSc, discusses signaling pathways and molecular mechanisms involved in the relapse of glioblastoma.

According to an expert from University Hospitals, studying pathways related to inflammation, epigenetics, and the microbiome may elucidate how patients with kidney cancer respond to anti-cancer therapy.
The addition of short-term androgen deprivation to dose-escalated radiation therapy did not yield a significant difference in quality-of-life outcomes vs radiotherapy alone for those with intermediate-risk prostate cancer, according to an expert from Henry Ford Health Cancer.
The novel radiotherapy Iomab-B prolongs survival and improves clinical outcomes among patients with relapsed/refractory acute myeloid leukemia, according to data from the phase 3 SIERRA trial.
James J. Driscoll, MD, PhD, and James Ignatz-Hoover, MD, PhD, share a perspective on a study published recently in ONCOLOGY.

“Even in well-resourced countries, there are disparities in exposure to treatments; some of these are rooted deeply in social determinants of health and represent a very complex problem of providing equal access.”
Data from the phase 2 TITAN-TCC trial suggest that early non-responders with metastatic urothelial carcinoma with PD-L1–positive tumors benefit from nivolumab plus nivolumab/ipilimumab boosts.
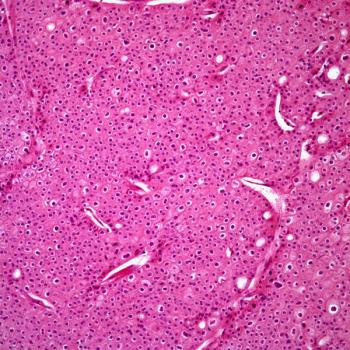
In the phase 3 RENOTORCH trial, frontline toripalimab plus axitinib appears to improve the overall response rate of patients with unresectable or metastatic renal cell carcinoma.
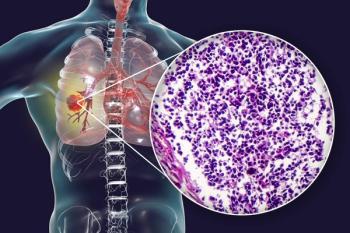
An update to the ASTRO and ESTRO clinical guidelines highlights the importance of a multidisciplinary approach to treating oligometastatic non–small cell lung cancer.

The impact of the gut microbiome, and antibiotics and probiotics on immunotherapy outcomes are areas of research currently under investigation for patients with kidney cancer and other cancers, according to an expert from University Hospitals.

Patients who use a recovery tracker tool appear to experience lower hospital readmission rates following gynecologic cancer debulking surgery compared with those who did not.
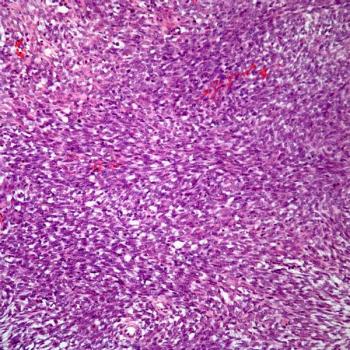
Findings from the phase 3 OUTBACK trial show that following standard cisplatin-based chemotherapy with adjuvant chemotherapy resulted in increased toxicity among patients with cervical cancer.