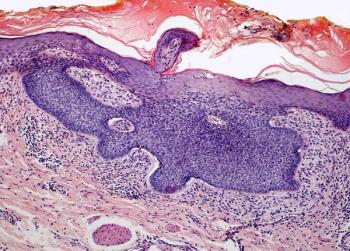
The 31-gene expression profile test appears to allow for significant and independent risk stratification of those with stage I cutaneous melanoma.

Your AI-Trained Oncology Knowledge Connection!


The 31-gene expression profile test appears to allow for significant and independent risk stratification of those with stage I cutaneous melanoma.
Results from a phase 2 study support additional clinical trials assessing belzutifan-based regimens for patients with advanced clear cell renal cell carcinoma.
Daniel V. T. Catenacci, MD, and colleagues present findings from a study of circulating tumor DNA as a predictive biomarker for gastric and gastroesophageal cancer.
Zhubin J. Gahvari, MD, MS, and Natalie S. Callander, MD, provide a comprehensive overview of current treatment paradigms in relapsed and refractory multiple myeloma.
Patients with curable metastatic cancer appear to have long-lasting improvements in depression symptoms 8 weeks after a single treatment with psilocybin therapy.
The safety profile of adagrasib monotherapy in advanced solid tumors harboring KRAS G12C mutations appears manageable, according to an expert from The University of Texas MD Anderson Cancer Center.

Medical oncologists and gynecologic oncologists alike have a shared responsibility to help treat symptoms of neuropathy in patients undergoing chemotherapy for gynecologic cancer, according to an expert from Duke University Medical Center.

Toripalimab plus chemotherapy appears tolerable in patients with resectable stage III non–small cell lung cancer, according to an expert from Shanghai Lung Cancer Center.

Co-editor-in-Chief Julie M. Vose, MD, MBA, writes about the effects and prevalence of burnout amongst oncologists and reviews strategies to address the issue.
The European Medicines Agency validates the potential indication of dostarlimab/chemotherapy in mismatch repair deficient/microsatellite instability–high endometrial cancer based on the phase 3 RUBY trial.
Investigators report that patients with advanced or metastatic esophageal squamous cell carcinoma may have superior overall survival benefit following treatment with tislelizumab/chemotherapy vs placebo/chemotherapy.

The phase 3 SPLASH trial will assess the benefit of 177Lu-PNT2002 in patients with PSMA-expressing metastatic castration-resistant prostate cancer.

Investigators cite the need for further study of the 25 mg, 3 times weekly exemestane dosing schedule in prevention studies among those with post-menopausal, stage 0 to II estrogen receptor–positive breast cancer who can’t tolerate daily adjuvant treatment.
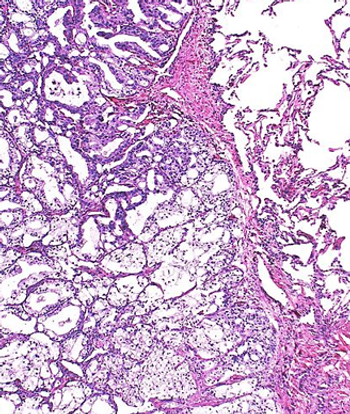
Data from the phase 3 MAGNITUDE study support the European Commission’s approval of niraparib plus abiraterone acetate dual action tablets in BRCA-mutated metastatic castration-resistant prostate cancer.

Experts from UCLA Health discuss key data presented at The Society of Gynecologic Oncology 2023 Annual Meeting on Women’s Cancer and how they may apply to clinical practice.

Adding fulvestrant to alisertib does not appear to improve objective in patients with endocrine-resistant metastatic breast cancer in the phase 2 TBCRC041 trial.

Future research assessing cryocompression for those with gynecologic cancers will make use of different products to make the intervention easier and more accessible for patients.

The FDA extends the Prescription Drug User Fee Act date to July 24, 2023 for quizartinib in the management of newly diagnosed FLT3-ITD mutation–positive acute myeloid leukemia.

An expert from University Hospitals touches on pain management guidelines that highlight moderate evidence in support of acupuncture, reflexology, and acupressure and massage as tools to manage general pain in patients with cancer.

Cryocompression demonstrates potential for preventing chemotherapy-induced neuropathy for those with gynecologic cancers, according to an expert from Duke University Medical Center.

Patients with locally advanced unresectable or metastatic gastric or gastroesophageal junction adenocarcinoma may experience significant survival benefit following treatment with zolbetuximab.
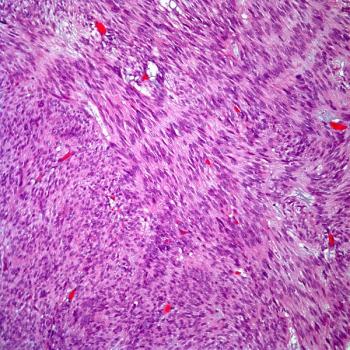
The safety profile of tislelizumab plus chemotherapy in the phase 3 RATIONALE 305 trial appears to be manageable in the treatment of advanced gastric or gastroesophageal junction adenocarcinoma.

Patients with platinum-resistant high-grade serous carcinoma have little overall survival benefit with the addition of vistusertib to weekly paclitaxel.

OTL78 appears to help in identifying prostate tumors, surgical margins, residual disease in the resection bed, and nodal metastases during PSMA-targeted fluorescence-guided surgery in those with PSMA-positive prostate cancer.
In older patients with hematologic malignancies undergoing hematopoietic stem cell transplantation, psychological distress at baseline was associated with worse quality of life outcomes.
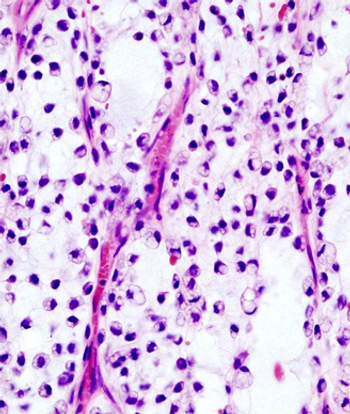
Patients with advanced well-differentiated/dedifferentiated liposarcoma may experience a positive progression-free rate following treatment with sitravatinib.

Investigators report that most patients with gynecologic cancers were able to tolerate cryocompression to help manage chemotherapy-induced neuropathy, according to an expert from Duke University Medical Center.

Patients with previously untreated diffuse large B-cell lymphoma can now receive polatuzumab vedotin-piiq plus rituximab, cyclophosphamide, doxorubicin, and prednisone following the FDA’s approval of the regimen.

Polatuzumab vedotin-piiq plus R-CHP can reduce the need for any subsequent lines of therapy for patients with diffuse large B-cell lymphoma, according to an expert from The University of Texas MD Anderson Cancer Center.

An expert from Dana-Farber Cancer Institute describes which patients hormone receptor-positive, HER2-negative breast cancer will benefit most from treatment with sacituzumab govitecan.