
Investigators say that liposomal irinotecan plus fluorouracil and leucovorin should be considered as a treatment option for second-line, previously treated metastatic biliary tract cancer.

Your AI-Trained Oncology Knowledge Connection!


Investigators say that liposomal irinotecan plus fluorouracil and leucovorin should be considered as a treatment option for second-line, previously treated metastatic biliary tract cancer.
Despite an improvement in disease-free survival, patients 60 years or over with acute myeloid leukemia do not appear to have a survival benefit from allo-HCT during first complete remission compared with consolidation chemotherapy.

The addition of bevacizumab to FOLFIRI does not appear to improve overall survival over FOLFIRI alone in patients with gastroenteropancreatic neuroendocrine carcinoma.

ALLO-316 has some “unique features” that may make it an “attractive” treatment option for those with kidney cancer, according to an expert from The University of Texas MD Anderson Cancer Center.

Data from the phase 3 SUNLIGHT study support a supplemental new drug application for trifluridine/tipiracil with or without bevacizumab for metastatic colorectal cancer.

Overall survival and progression-free survival appear comparable in patients with relapsed small cell lung cancer treated with liposomal irinotecan vs topotecan.
Investigators will assess botensilimab and balstilimab for non-microsatellite instability-high/mismatch repair deficient metastatic colorectal cancer in a randomized phase 2 trial.

Corey Cutler, MD, MPH, FRCPC, discusses how patients with blood cancer in need of an umbilical cord blood transplant may benefit from omidubicel-onlv.

The FDA expresses openness towards having protocol discussions with investigators on initiating a second clinical study evaluating synthetic hypericin sodium for cutaneous T-cell lymphoma.

An expert from University Hospitals highlights different guidelines penned by oncology organizations informing the integrative management of symptoms including anxiety and depression in patients with kidney cancer.

Patients with hematologic malignancies who require allogenic hematopoietic stem cell transplant can now receive omidubicel following the FDA’s approval of the agent.
Data from the phase 3 KarMMA-3 trial support several applications for idecabtagene vicleucel in earlier use for triple-class exposed multiple myeloma in the United States, Europe, and Japan.

Fareed Khawaja, MBBS, and Marilyne Daher, MD discuss COVID-19 vaccine efficacy and safety among patients with cancer in the United States.

An expert from Dana-Farber Cancer Institute highlights the unmet needs that sacituzumab govitecan meets in the treatment of advanced hormone receptor-positive, HER2-negative breast cancer.

Massage and acupuncture represent promising integrative care strategies for managing stress and pain in patients with kidney cancer and other tumors, according to an expert from University Hospitals.

A senior physician’s assistant from Johns Hopkins examines why adverse effect management is so crucial and reviews how to help maintain quality of life while undergoing treatment.

Findings from a prospective study suggest that circulating tumor DNA sequencing with a large panel may efficiently identify actionable alterations in patients with advanced solid tumors.

Lifestyle choices such as diet and exercise can be useful in managing symptom burden and may even have an impact on some treatment outcomes, according to a director of Lifestyle Medicine from Massachusetts General Hospital.

OM-301, which demonstrated preclinical anti-cancer activity in multiple myeloma, receives orphan drug designation from the FDA.

Adding pembrolizumab to chemotherapy with or without bevacizumab can help patients with persistent, recurrent, or metastatic cervical cancer “live longer and better,” according to an expert from the University of Arizona College of Medicine.

In addition to the benefit seen with hormone therapy plus metastasis-directed radiation in oligometastatic prostate cancer, use of intermittent hormone therapy may result in positive disease control and longer eugonadal testosterone intervals.

Investigators highlight the importance of building partnerships between CAR T-cell centers and external hospitals to increase treatment referrals and access to clinical trials for patients with B-cell acute lymphoblastic leukemia.

The FDA sets a Prescription Drug User Fee Act date of December 16, 2023 for pembrolizumab plus chemotherapy for managing metastatic gastric or gastroesophageal junction adenocarcinoma.

Incorporating nursing-led palliative care may help to improve advanced care planning among those with advanced cancer, especially those without access to specialty palliative care.

An expert from Dana-Farber Cancer Institute highlights data that supported the FDA’s approval of sacituzumab govitecan for advanced hormone receptor–positive, HER2-negative breast cancer.

Krina Patel, MD, MSc, discusses research and initiatives that may help to mitigate disparities in patients with multiple myeloma including factors such as gender, race, and ethnicity.
Data from the interim analysis of the phase 3 FLAMES study indicate that senaparib may lengthen progression-free survival in patients with advanced ovarian cancer.
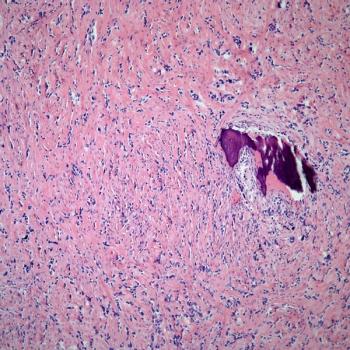
TP-1287 is under investigation as part of a phase 1 study in patients with metastatic or progressive solid tumors, including Ewing sarcoma, that are refractory or intolerant to other established therapies.
There is a clear need for further trials and drug development to mitigate poor outcomes in patients with small cell lung cancer, according to an expert from Yale School of Medicine.

CB-011, which received fast track designation from the FDA, is currently under investigation as a treatment for those with relapsed or refractory multiple myeloma in the phase 1 CaMMouflage trial.