
The use of palliative care in ovarian cancer resulted in a decrease in overall readmissions and index hospitalization costs.

Your AI-Trained Oncology Knowledge Connection!


The use of palliative care in ovarian cancer resulted in a decrease in overall readmissions and index hospitalization costs.
Durvalumab plus tremelimumab in combination with neoadjuvant chemotherapy did not yield any major adverse effects in patients with newly diagnosed advanced ovarian cancer in the phase 2 KGOG3046 trial.
Minimally invasive surgery for interval debulking resulted in a lower mortality at the 30- and 90-day time points compared with laparotomy in advanced ovarian cancer.
Vadim Gushchin, MD, says that treatment with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy should not be limited by concerns of negatively impacting health-related quality of life in ovarian cancer.

Current clinical trials look to assess 177Lu-PSMA-617 in combination with other therapies including androgen deprivation therapy and docetaxel.

An expert from Dana-Farber Cancer Institute indicates that patients with prostate cancer who have 1 risk factor should undergo salvage radiotherapy following radical prostatectomy before their prostate-specific antigen level rises above 0.25 ng/ml.

An expert panel highlights novel therapeutic approaches for newly diagnosed and relapsed multiple myeloma.
Findings from 2 clinical trials support the approval of acalabrutinib as a treatment for patients with mantle cell lymphoma in China.
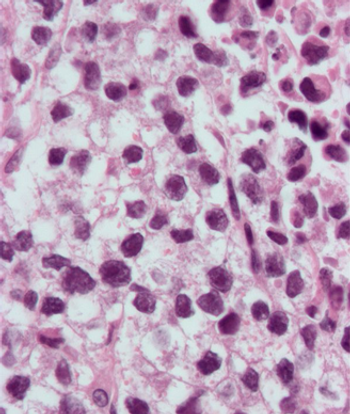
Treatment with retroperitoneal lymph node dissection for testicular seminoma with limited retroperitoneal lymphadenopathy is associated with low long-term morbidity.
Jing Han, MD, and colleagues examine the prevalence and characteristics of ocular toxicities associated with MEK inhibition.
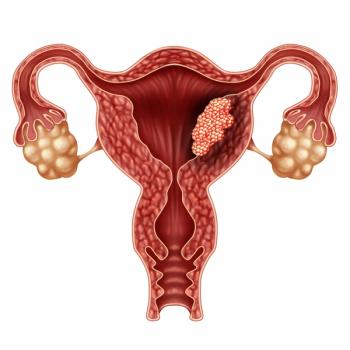
Adding avelumab to carboplatin plus paclitaxel appears to elicit a progression-free survival advantage compared with chemotherapy alone in those with advanced or recurrent endometrial cancer.
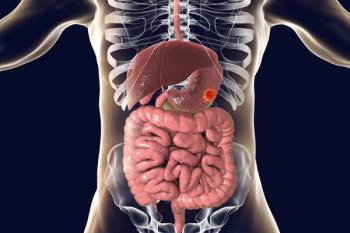
Efficacy and safety findings from the phase 3 GLOW trial assessing zolbetuximab and CAPOX in CLDN18.2-positive locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma were consistent with the phase 3 SPOTLIGHT trial, according to an expert from Weill Cornell Medical College
Javier Orozco-Mera, MD, FACS, MSc, and colleagues assess the signaling pathways and molecular mechanisms involved in the relapse of glioblastoma.

Patients with metastatic or recurrent locally advanced Merkel cell carcinoma can now receive treatment with retifanlimab-dlwr following its accelerated approval by the FDA.

Findings from a cohort of the Childhood Cancer Survivor Study identify a prediction model that may accurately identify childhood cancer survivors at varying risks of late kidney failure.
Human papillomavirus and p16 discordance may correlate with a worse prognosis for oropharyngeal cancer, according to data from an individual patient data analysis.
Patritumab deruxtecan continues to yield positive efficacy in 2 early phase trials assessing the agent in patients with metastatic non–small cell lung cancer and breast cancer.

An expert from Weill Cornell Medicine highlights key clinical data indicating the benefits of radium-223 in the treatment of patients with metastatic castration-resistant prostate cancer.

A genetic analysis indicates that multi-ancestry polygenic risk scores may be “potentially useful” in detecting risk of aggressive prostate cancer in patients of African ancestry, according to an expert from the University of Southern California.
According to phase 1b/2 HPV001 study data, VTP-200 appears to produce no serious adverse effects in patients with low-grade cervical human papillomavirus lesions.
Investigators plan to submit a communication application to seek marketing approval in China for ARX788 as therapy for HER2-positive advanced or metastatic breast cancer.
Investigators are assessing FORE8394, which received orphan drug designation from the FDA, in primary brain and central nervous system malignancies in the phase 2 FORTE trial.
Combination treatment with nivolumab and relatlimab appears to be a safe treatment option for patients with advanced melanoma who have progressed on prior anti–PD-L1 therapy.

Data from the phase 2 INNATE trial indicate that pimivalimab plus JTX-8064 also appears to be well tolerated in patients with platinum-resistant ovarian cancer.
Bria-IMT plus retifanlimab continues to show positive clinical outcomes in a small cohort of patients with advanced, metastatic breast cancer, according to data from a phase 1/2 trial.
Enzalutamide plus leuprolide appears to improve metastases-free survival vs placebo plus leuprolide in patients with non-metastatic hormone-sensitive prostate cancer.

The risk of radionuclide exposure to the public reflects one reason urologists need to collaborate with radiation oncologists when administering radiopharmaceuticals to patients with prostate cancer.
The phase 3 LIMBER-304 trial examining parsaclisib with ruxolitinib vs matched placebo will be discontinued after an interim analysis concluded it would likely miss the primary end point.

Switching out beta emitters for alpha emitters, including radium-223, is one way to improve radiopharmaceutical treatment of prostate cancer, according to an expert from Weill Cornell Medicine.
New findings from the phase 3 ADAURA trial “provide powerful evidence” of osimertinib’s potential to extend the lives of patients with early-stage, EGFR-mutant non–small cell lung cancer, according to an expert from Yale Cancer Center.