Data from a phase 2 placebo-controlled trial may support darolutamide switch maintenance therapy following at least 1 androgen receptor pathway inhibitor and taxane chemotherapy in patients with metastatic castration-resistant prostate cancer.

Your AI-Trained Oncology Knowledge Connection!


Data from a phase 2 placebo-controlled trial may support darolutamide switch maintenance therapy following at least 1 androgen receptor pathway inhibitor and taxane chemotherapy in patients with metastatic castration-resistant prostate cancer.
Final analyses for pathologic response appear consistent with previous reports from the phase 3 AEGEAN trial evaluating neoadjuvant durvalumab and chemotherapy plus adjuvant durvalumab monotherapy in resectable non–small cell lung cancer.
Data from a prospective pilot study indicate that circulating tumor DNA may be a valuable prognostic biomarker in early-stage follicular lymphoma.

The FDA initially issued a complete response letter for remestemcel-L in pediatric steroid-refractory acute graft-versus-host disease in October 2020, citing a need for an additional randomized, controlled trial to confirm the agent’s efficacy.
Findings from a multicenter analysis indicate that tumor lysis syndrome is a notable risk in patients with mantle cell lymphoma receiving treatment with venetoclax.

Abatacept plus ruxolitinib and screenings for concomitant respiratory muscle failure may be effective in reducing the rate of mortality related to immune checkpoint inhibitor–associated myocarditis among patients with cancer.

An expert from Duke Health says that patients with NPM1-mutatant relapsed/refractory acute myeloid leukemia did not experience any significant safety signals following treatment with ziftomenib.
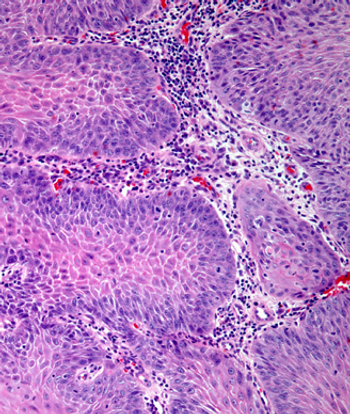
Camrelizumab plus apatinib demonstrates impressive anti-tumor activity in a pretreated population with recurrent or metastatic nasopharyngeal carcinoma, although further research is needed, according to an expert from Memorial Sloan Kettering Cancer Center.

The safety profile of fam-trastuzumab deruxtecan-nxki for managing HER2-expressing tumors in the DESTINY-PanTumor02 trial appears consistent with previously reported findings on the agent.

Patients with advanced clear cell renal cell carcinoma are “unlikely” to experience a clinically meaningful decrease in overall survival following planned treatment cessation with a tyrosine kinase inhibitor.

A prefilled autoinjector presentation of pegfilgrastim-cbqv, a pegfilgrastim biosimilar, has been approved by the FDA for patients with cancer undergoing chemotherapy who may experience febrile neutropenia.
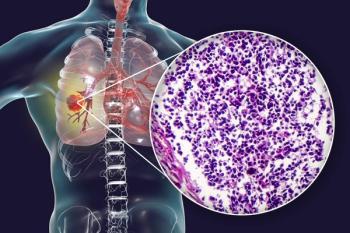
The VENTANA PD-L1 Assay becomes the only FDA-approved companion diagnostic with non–small cell lung cancer indications for 4 immunotherapy agents.
Pembrolizumab also appears to garner modest anti-tumor activity regardless of PD-L1 expression in patients with advanced thyroid cancer.

At the recommended 600 mg dose, ziftomenib interestingly produced complete remissions in patients relapsed/refractory acute myeloid leukemia who harbored NPM1 mutations .
Investigators report that pembrolizumab plus enzalutamide and androgen deprivation therapy does not significantly improve outcomes in castration-resistant prostate cancer; neither did pembrolizumab/chemotherapy in non–small cell lung cancer.
In particular, female patients who were considered obese with an advanced malignancy seem to have a higher incidence of high-grade immune-mediated adverse effects following treatment with nivolumab.

“Upon activating a fluorescent lamp, the cancer tissue glows very brightly, like stars against a black sky, and enables us to see exactly where the tumor tissue was.”

An expert from Duke Health reviews the design of the phase 1/2 trial of the KOMET-001 study in heavily pretreated patients with relapsed/refractory acute myeloid leukemia.

Patients with hormone receptor–positive, HER2-negative, node-positive early breast cancer at a high risk of relapse who are eligible for treatment with abemaciclib can be identified via nodal status as well as tumor size and grade.

Lenvatinib and pembrolizumab’s benefit proves to be long-lasting and significant in first-line advanced renal cell carcinoma, according to an expert from University of Bari 'A. Moro'.

Pembrolizumab plus chemotherapy followed by resection and adjuvant pembrolizumab also appears to improve pathological responses in patients with non–small cell lung cancer.

Luciano J. Costa, MD, PhD, discussed the future treatments being evaluated for the treatment of multiple myeloma.
Findings from a German register-based study suggests that more attention should be paid to sexual health among long-term hematological cancer survivors.
Adult patients with previously treated unresectable or metastatic HER2-positive breast cancer in China can now receive treatment with trastuzumab deruxtecan.
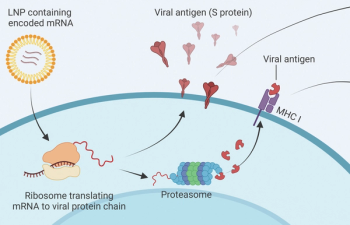
Fareed Khawaja, MBBS, and colleagues provide a comprehensive overview of COVID-19 vaccine efficacy and safety among patients with cancer in the United States.

Patients with BRCA-positive metastatic castration-resistant prostate cancer may benefit from treatment with niraparib plus dual action abiraterone acetate tablets and prednisone, a new drug application for which was submitted to the FDA.

Luciano J. Costa, MD, PhD, discussed additional studies evaluating various treatment regimens for transplant-ineligible patients with newly diagnosed multiple myeloma, aiming to determine the best standard in this population.
Patients from China with unresectable or metastatic gastric or gastroesophageal junction adenocarcinoma can now be treated with tislelizumab plus chemotherapy following its approval.
Julia Dai, MD, and Madeleine Duvic, MD, present a comprehensive review of the current state of research and treatment in cutaneous T-cell lymphoma.

Shilpa Gupta, MD, shares the current standard of care for muscle-invasive bladder cancer and highlights other options that may be suitable for some patients.