
The phase 3 LIMBER-304 trial examining parsaclisib with ruxolitinib vs matched placebo will be discontinued after an interim analysis concluded it would likely miss the primary end point.

Your AI-Trained Oncology Knowledge Connection!


The phase 3 LIMBER-304 trial examining parsaclisib with ruxolitinib vs matched placebo will be discontinued after an interim analysis concluded it would likely miss the primary end point.

Switching out beta emitters for alpha emitters, including radium-223, is one way to improve radiopharmaceutical treatment of prostate cancer, according to an expert from Weill Cornell Medicine.

New findings from the phase 3 ADAURA trial “provide powerful evidence” of osimertinib’s potential to extend the lives of patients with early-stage, EGFR-mutant non–small cell lung cancer, according to an expert from Yale Cancer Center.

Data demonstrate the feasibility of automated glomerular filtration rate prediction to decide between partial nephrectomy and radical nephrectomy in kidney cancer, according to an expert from the Cleveland Clinic.

A panel of experts reviews how the recent approval of teclistamab, a bispecific antibody, has changed the treatment landscape for patients with multiple myeloma.
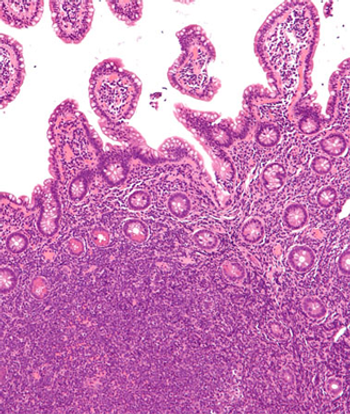
Investigators indicate that although avoiding efficient mantle cell lymphoma treatment due to late effects may seem okay, most long-term health care needs are disease related in patients up to 70 years.

Results from the phase 2 CDRB436G2201 trial lead to the approval of dabrafenib plus trametinib in patients with low-grade glioma and a BRAF V600E mutation who require systemic therapy.

Use of a kit that prepares for gallium Ga 68 gozetotide injection in the phase 3 VISION study affirms its ability to identify patients who are suitable to receive PSMA-based radioligand for metastatic prostate cancer.
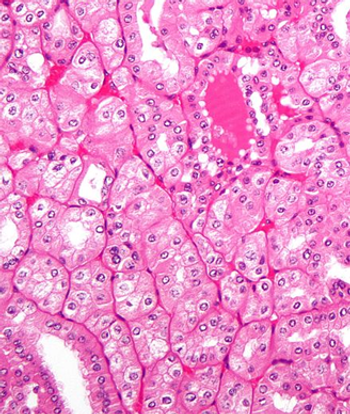
Kidney Cancer Association president Gretchen Vaughan discusses improvements being rolled out by the organization and the importance of multidisciplinary care.

Findings from a confirmatory factor analysis appear to validate the structure of the Quality of Life in Myelodysplasia Scale in myelodysplastic syndromes.

National Comprehensive Cancer Network guidelines will now include ripretinib as the ideal second-line treatment for patients with unresectable/metastatic gastrointestinal stromal tumors who are intolerant to sunitinib.

Early phase trials investigating cellular therapies, bispecific antibodies, and antibody-drug conjugates for refractory kidney cancer may uncover strategies to overcome resistance mechanisms.
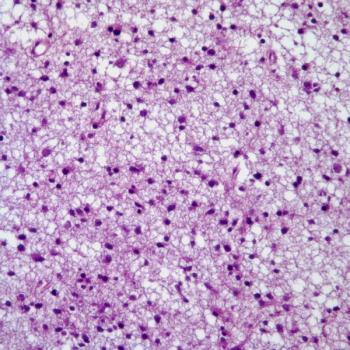
Vorasidenib monotherapy appears to improve clinical outcomes among patients with IDH-mutant low-grade glioma, meeting 2 end points in the phase 3 INDIGO trial.

Factors including age, gender, and facility type may influence the likelihood of pain specialist referrals for lung cancer, suggesting a need for multidisciplinary pain management in this patient population.
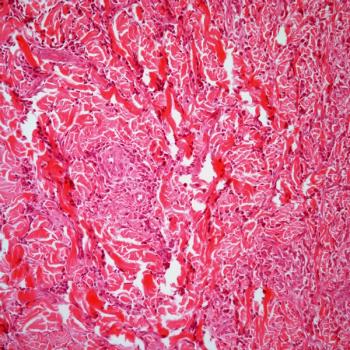
EP0042 shows promising early clinical efficacy in patients with pretreated acute myeloid leukemia, according to investigators.

Anthony D’Amico, MD, PhD, reviewed current practices and unanswered questions surrounding radiotherapy in the realm of prostate cancer.

Increasing cancer antigen presentation as well as working with tumor cells in and delivering novel cells to the microenvironment may help in overcoming mechanisms of immune checkpoint inhibitor resistance in refractory renal cell carcinoma.

Matthew Dallos, MD, discusses current challenges in the treatment of castration-resistant prostate cancer and the potential of antibody-drug conjugates in this disease space.

The addition of bortezomib to dexamethasone plus rituximab and cyclophosphamide appears to improve progression-free survival among those with Waldenström Macroglobulinemia.

Investigators indicate that strategies are necessary to identify patients with extensive-stage small cell lung cancer who won’t benefit from immunotherapy-based treatment.

Shilpa Gupta, MD, discusses bladder preserving strategies for unfit or young patients, trials assessing immunotherapy combinations, and results from the BLASST-1 trial presented at ASCO GU.

Nirogacestat may be a noteworthy therapeutic advance for patients with desmoid tumors, according to an expert from Memorial Sloan Kettering Cancer Center.

The final analysis of the phase 2/3 CCTG IND.227/KEYNOTE-483 trial identifies a statistically significant survival benefit with pembrolizumab and chemotherapy vs chemotherapy alone in the first-line setting for patients with unresectable advanced or metastatic malignant pleural mesothelioma.

Lenvatinib plus pembrolizumab appears to be the best option for patients with refractory metastatic renal cell carcinoma who are progressing on immunotherapy combinations or are lenvatinib naïve.

Ipilimumab monotherapy does not appear effective in driving complete responses in refractory renal cell carcinoma despite yielding some progression-free survival intervals, according to an expert from the University of Texas Southwestern Medical Center.

Disparities in pediatric cancer survival in Europe might be mitigated through cross-border care, twinning programs, and use of pan-Europe cancer control plans.

The presence of teratoma in pretreatment non-seminomatous germ-cell tumors correlates with a higher risk of teratomatous elements in residual non- retroperitoneal disease following chemotherapy.

An expert from the University of Texas Southwestern Medical Center discusses several phase 3 clinical trials supporting the use of various single-agent and combination immunotherapy regimens for advanced kidney cancer.

All patients with platinum-resistant, small-cell neuroendocrine prostate cancer who responded to treatment with BXCL701 plus pembrolizumab were microsatellite stable and/or tumor mutational burden–low with a low probability of response to pembrolizumab.

Polatuzumab vedotin-piiq plus R-CHP was found to produce clinical efficacy in phase 3 POLARIX trial in patients with previously untreated diffuse large B-cell lymphoma, leading to an 11-to-2 vote from the FDA’s Oncologic Drug Advisory Committee.