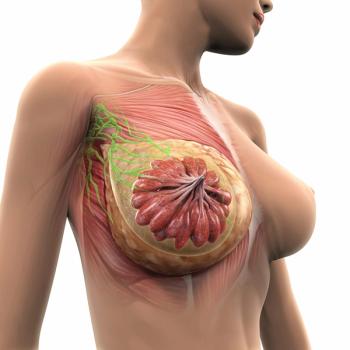
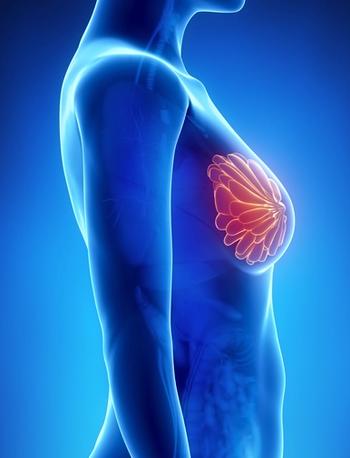
Dexamethasone, Cisplatin, gemcitabine, and pegaspargase resulted in improved preliminary outcomes vs dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide for patients with newly diagnosed locally advanced stage III or IV extranodal natural killer/ T-cell lymphoma.