A research team from the Mayo clinic verified the known risk factors for endometrial cancer, while identifying a possible microbiome signature associated with the disease.

Your AI-Trained Oncology Knowledge Connection!


Dostarlimab Shows Promising Antitumor Activity in Mismatch Repair Deficient, Proficient Endometrial Cancer

A research team from the Mayo clinic verified the known risk factors for endometrial cancer, while identifying a possible microbiome signature associated with the disease.
Patients with endometrial cancer pose a very high risk of dying from cardiovascular diseases in the year after diagnosis, suggesting the necessity of early involvement of cardiologists for these patients.
Endometrial cancer survival correlates with total number of chemotherapy cycles – but not whether they are started earlier than radiation, according to the results of a new study.

A recent Danish study showed pregnancy loss was not associated with later cancer development among women with various cancer types.
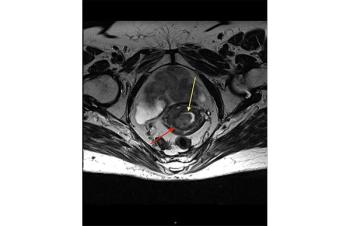
COX7RP contributes to endometrial and breast cancer growth.

Diagnosis of endometrial carcinoma made easier by analysis of mutations in cervix or endometrial tissue.
Many clinical trials in breast and endometrial cancers are underway and recruiting patients.

The combination was simultaneously approved in Australia and Canada and was the first such action under a new international collaboration.
Researchers identified a highly recurrent, disease-specific PP2A PPP2R1A mutation as a driver of endometrial carcinoma and as a target for new drugs.
Researchers examined the results of ribociclib and letrozole as a treatment for patients with estrogen receptor-positive ovarian cancer and endometrial cancer.
A new study reinforces the need for universal mismatch repair protein immunohistochemistry screening of tumors from endometrial cancer patients, as well as germline genetic testing in accordance with guidelines.

Dr. Makker summarizes the standard of care for the treatment of advanced endometrial cancer, with a look forward to the multitude of clinical trials evaluating novel therapy combinations that are currently enrolling patients or underway.

Cancer Network spoke with Yoland Catherine Antill, MD, of Cabrini Health, about the phase II PHAEDRA trial, which tested the activity of durvalumab in advanced endometrial cancer according to mismatch repair status.
Researchers studied the use of minimally invasive robotic surgery for hysterectomy and its risk for complications in the treatment of endometrial cancer in Denmark.

Adjuvant radiation therapy provides a local control benefit in stage III endometrial carcinoma patients, as strongly suggested in retrospective and prospective series.

The routine use of adjuvant radiation therapy in stage III endometrial cancer patients is not supported by the current literature and should not be employed universally in this group of patients.

A meta-analysis finds that postmenopausal bleeding occurs in approximately 90% of women with endometrial cancer.

The combination of the PARP inhibitor olaparib with the mTORC1/2 inhibitor vistusertib had promising activity across endometrial, ovarian, and triple-negative breast cancers.

Researchers discovered that estrogen receptor alpha and glucocorticoid receptor are simultaneously expressed in many endometrial cancers, and may be associated with poor prognosis.

Findings from the GOG-3007 study suggest everolimus plus letrozole might be effective in advanced persistent or recurrent endometrial carcinoma.

Use of oral contraceptives may be beneficial for chemoprevention for a range of women with different baseline cancer risks, according to the results of a new study.

Two studies on Lynch syndrome highlight cancer screening and surveillance opportunities.

Women with increased levels of cadmium, which mimics estrogen in the body, had a higher risk of endometrial cancer, according to a recently published observational study.

The diagnostic benefits of SLN evaluation include an ability to identify the extent of tumor dissemination and the utility of SLN mapping in guiding targeted adjuvant treatment in high-risk patients.

The question that should be posed to those who advocate for lymphadenectomy as a tool to guide the adjuvant therapy of endometrial cancer is: At what cost to the patient is lymphadenectomy performed?