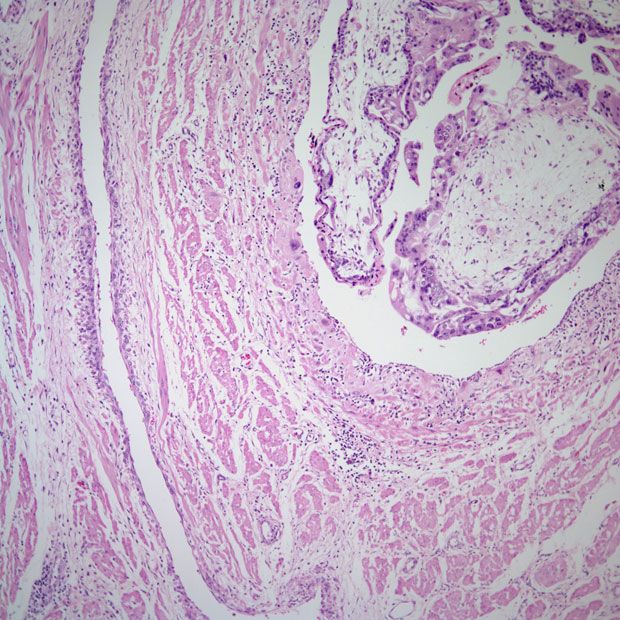
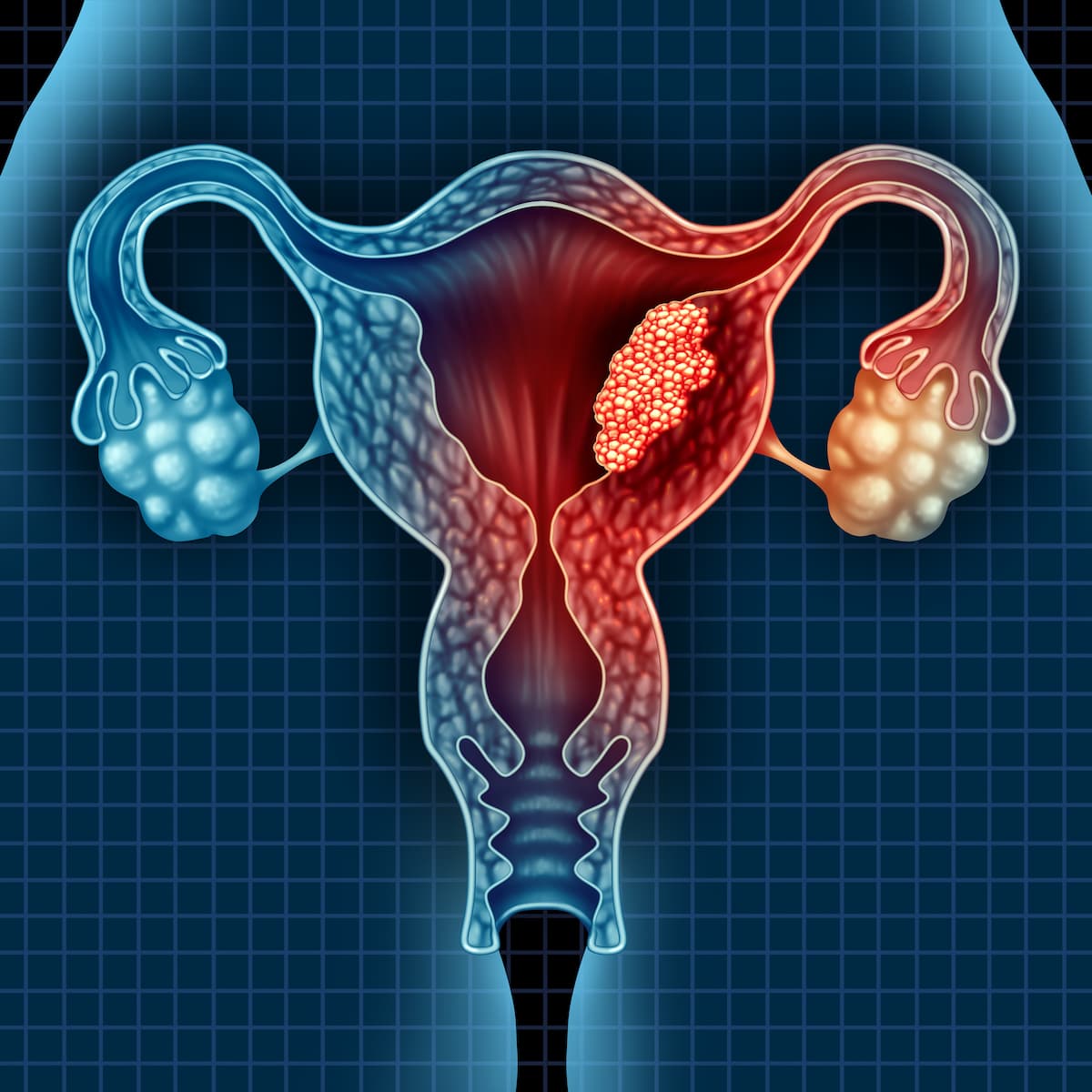
Endometrial Cancer
Latest News

Data Suggest Worse Survival in Black Low-Risk Endometrial Cancer Population
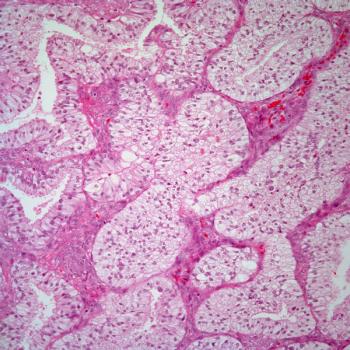
Endometrial Cancer Molecular Classification May Predict Radiotherapy Response
Latest Videos

CME Content
More News
A progression-free survival benefit was seen in those with TP53 wild-type advanced/recurrent endometrial cancer who were treated with selinexor maintenance regardless of microsatellite instability status.

The QPOLE assay may be a fast, low-cost alternative to other next-generation sequencing tools for POLE testing among patients with endometrial cancer.
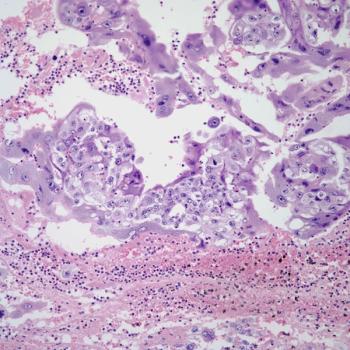
Pembrolizumab plus chemotherapy may improve clinical outcomes over placebo among patients with advanced or recurrent endometrial cancer.

Ritu Salani, MD, details the health-related quality of life benefits associated with dostarlimab in the treatment of advanced endometrial cancer, which includes improvements in back and pelvic pain.

Ritu Salani, MD, describes the concordance between blinded independent central review and provider-assessed outcomes with dostarlimab among patients with advanced recurrent endometrial cancer in the phase 3 RUBY trial.

The panel explains how low-grade serous ovarian cancer differs from high-grade, and if an immunotherapy approach is appropriate for treatment.

Robert Coleman, MD, FACOG, FACS, reviews data from a study investigating combination letrozole plus ribociclib in patients with low-grade serous ovarian cancer.
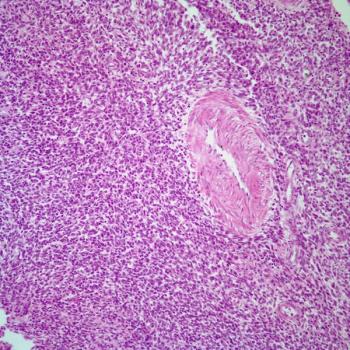
The FDA accepts a supplemental biologics license application for dostarlimab plus chemotherapy in the treatment of mismatch repair deficient/microsatellite instability-high advanced or recurrent endometrial cancer based on data from a prespecified interim analysis of the phase 3 RUBY/ENGOT-EN6/GOG3031/NSGO trial.

Health-related quality of life data support dostarlimab plus chemotherapy as a standard of care in primary advanced or recurrent endometrial cancer, according to an expert from Copenhagen University Hospital in Denmark.

Dostarlimab produces a survival benefit vs placebo in combination with standard of care therapy regardless of whether patients had mismatch repair deficient/microsatellite instability-high disease.

Drs Eskander and Lewin discuss the sequencing immune checkpoint inhibitors for women with recurrent endometrial cancer.

Sharyn Lewin, MD, FACS, shares how she discusses side effects with patients receiving immune checkpoint inhibitors for advanced/recurrent endometrial cancer.

Data from the phase 3 DUO-E trial indicate that the safety of durvalumab/chemotherapy with or without olaparib/durvalumab or durvalumab monotherapy maintenance in recurrent endometrial cancer was consistent with previous reports of each agent.

Experts explain what they are looking forward to in the future of treatment for patients with ER/PR+ and P53 wild-type or mutated advanced/recurrent endometrial cancer.

The panel discusses their opinions on the use of immunotherapy in different populations of women with advanced/recurrent endometrial cancer.

Prexasertib is currently under investigation as part of a phase 2 trial as a treatment for patients with platinum-resistant ovarian cancer, endometrial adenocarcinoma, and urothelial cancers.
Closing the discussion on advanced endometrial carcinoma, Dr David O’Malley shares his perspective on the future treatment landscape.

The European Medicines Agency validates the potential indication of dostarlimab/chemotherapy in mismatch repair deficient/microsatellite instability–high endometrial cancer based on the phase 3 RUBY trial.

Dostarlimab plus chemotherapy appears to improve progression-free survival vs placebo plus chemotherapy in patients with recurrent endometrial cancer in the phase 3 RUBY trial.

Pembrolizumab plus chemotherapy followed by maintenance pembrolizumab reduced the risk of death or disease progression in patients with mismatch repair proficient or deficient advanced endometrial cancer.
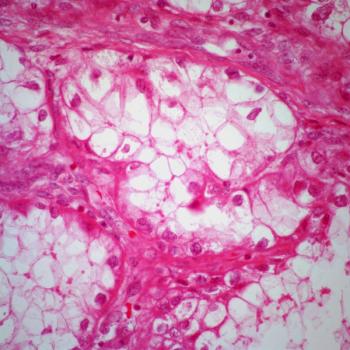
Adavosertib does not appear to be well tolerated in patients with previously treated recurrent or persistent uterine serous carcinoma in the phase 2b ADAGIO trial.
The phase 3 RUBY trial of dostarlimab plus carboplatin and paclitaxel significantly improved progression-free survival in patients with dMMR/MSI-H or MMRp/MSS endometrial cancer.

Patients with advanced endometrial cancer experience early responses to lenvatinib plus pembrolizumab in both patients who were mismatch repair proficient and all-comers.

In the SOLAR phase 1b trial, a clinical benefit was observed when patients with RAS-mutated ovarian or endometrial cancer were given olaparib plus selumetinib.

The lower extremity lymphedema screening questionnaire and gynecological cancer lymphedema questionnaire appear to demonstrate comparable utility in assessing lymphedema in patients with advanced endometrial cancer.