
Researchers have shown that patients who use nonsteroidal anti-inflammatory drugs like aspirin and ibuprofen are less likely to develop three types of skin cancer.

Your AI-Trained Oncology Knowledge Connection!



Researchers have shown that patients who use nonsteroidal anti-inflammatory drugs like aspirin and ibuprofen are less likely to develop three types of skin cancer.

A new analysis of four large European trials of cutaneous melanoma patients showed a substantial survival advantage for women, most likely explained by underlying biologic differences.
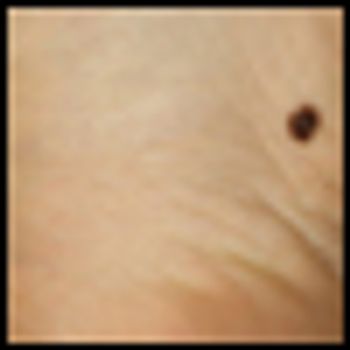
The addition of sorafenib to isolated limb infusion therapy with melphalan did not improve responses in a new phase I trial of patients with in-transit extremity melanoma.
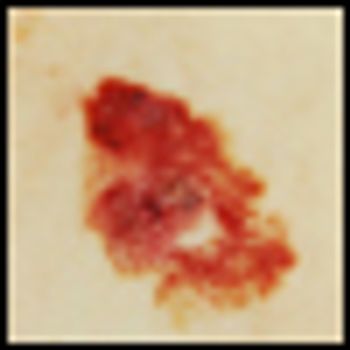
Melanoma has become the poster cancer for genomic research. The identification of a driver mutation in the BRAF gene found in approximately 40% of metastatic melanoma patients and the subsequent approval last year of the targeted BRAF inhibitor, vemurafenib, has resulted in a surge of both clinical and laboratory research.
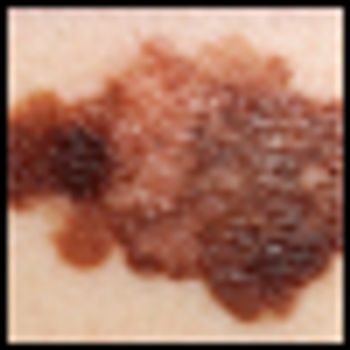
The presence of a mutation in the MEK1 gene in melanoma patients does not cause resistance to BRAF inhibitor therapy in patients that also carry a BRAF mutation, according to a new study. Previously, experts believed that resistance to the drugs in BRAF-mutated melanoma patients could likely be blamed on the concurrent mutation in MEK1.
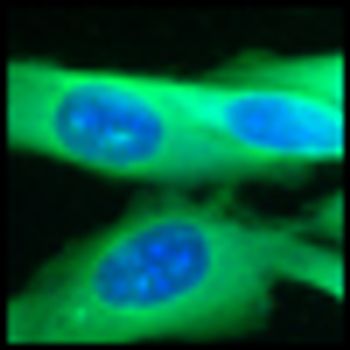
Researchers at the Mayo Clinic in Rochester, Minnesota, found an eight-fold increase in melanoma incidence among young women between 1970 and 2009 in an epidemiological study. The increase was not as striking among young men, but there was still a four-fold jump in melanoma cases over those four decades.
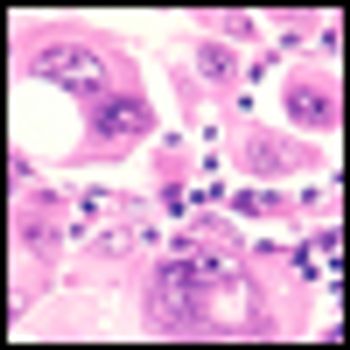
Results from a phase II trial of vemurafenib for metastatic melanoma show a significant number of patients exhibiting a prolonged and durable response and a median overall survival of 16 months.
A study published last month shows that the melanoma drug ipilimumab has activity in melanoma patients whose cancer has spread to the brain. Long-term survival of melanoma patients with brain metastases was comparable to survival of metastatic melanoma patients without brain metastases.

A study published this week shows that taking retinol, a form of vitamin A, results in a decrease in the risk of developing melanoma. The effect is limited to those who took vitamin A in excess of standard multivitamin guidelines and was more pronounced in women than in men.

The US Food and Drug Administration (FDA) announced the approval of vismodegib (Erivedge), for the treatment of advanced basal cell carcinoma, the most common type of skin cancer, for patients who are not eligible for surgery or radiation, and for metastatic disease.

Metastatic melanomas that harbor the V600E mutation in the BRAF gene respond rapidly to vemurafenib (Zelboraf), the BRAF V600E inhibitor. But While vemurafenib results in a response in about 80% of melanoma patients, the clinical response among CRC patients is not greater than 5%.
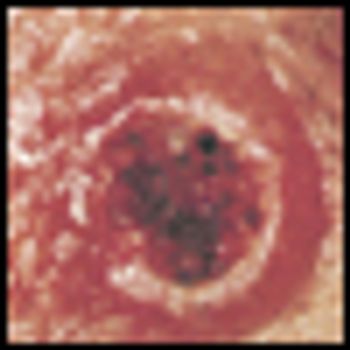
In a rare case where the side effect of a cancer treatment can be explained through molecular studies, researchers have identified the reason behind the frequent skin lesion side effect among metastatic melanoma patients that take the newly approved drug vemurafenib.

A large cohort study shows that women on antiestrogen therapy have a lower risk of melanoma. In a study of 7360 women diagnosed with breast cancer between 1980 and 2005, 54% were given supplemental antiestrogen therapy. The rate of cutaneous melanoma was 60% higher for those women not taking antiestrogen supplements compared with the expected rate of melanoma incidence based on age and other factors.

The management of in-transit metastases is challenging, since the treatments and extent of disease vary greatly based on the number, depth, location, and distribution of lesions, and on their biological behavior.

The past year in oncology was highlighted by the continuation of breakthroughs in targeted therapies-with new treatments receiving US Food and Drug Administration (FDA) approval for non–small-cell lung cancer (NSCLC), lymphoma, and melanoma.

For localized in-transit disease, less is more, with local destruction, excision, and intralesional therapy being the cornerstones of treatment. If local therapies fail or if distant disease arises, isolated limb perfusion and systemic therapy remain effective options.

While patients with in-transit disease represent a wide spectrum of disease that requires individualized therapy, great opportunity exists to learn from our treatment interventions in these individuals.

Immunotherapy is finally getting the cancer clinical and research community excited: A large portion of the presentation and discussion at the Melanoma International Congress, held in Tampa, Florida last week focused on immunotherapy approaches for the treatment of the disease.

The theme of the SMR meeting in Tampa this year was “Advancement through Collaboration," and this theme was clearly reflected in the meeting.

Resection of liver metastases represents a major advance of the last few decades in the treatment of colorectal cancer.

Hepatic metastases remain a lethal and recalcitrant problem in the management of malignant disease, and the review by Drs. Zani and Clary of the role of hepatic metastasectomy for patients with stage IV melanoma or breast cancer is timely and welcome.

Flax, an annual plant believed to have originated in Egypt, is cultivated around internationally and is among the world’s oldest crops.

This review summarizes the existing literature that addresses the topic of metastasectomy in patients with melanoma and breast cancer.

With positive read-outs from trials over the last two years and approval of two new agents for the disease, Yervoy and Zelboraf, the field is already looking to new agents and combination trials to improve patient outcomes and survival.
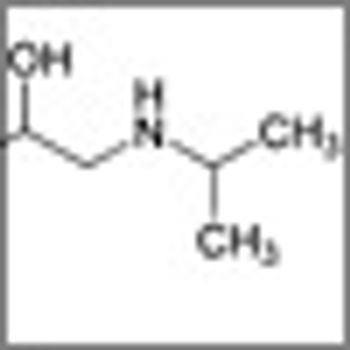
Researchers have shown that the use of β-adrenoceptor antagonists (β-blockers) for 1 year or more is associated with a decreased risk of progression of thick malignant melanoma.