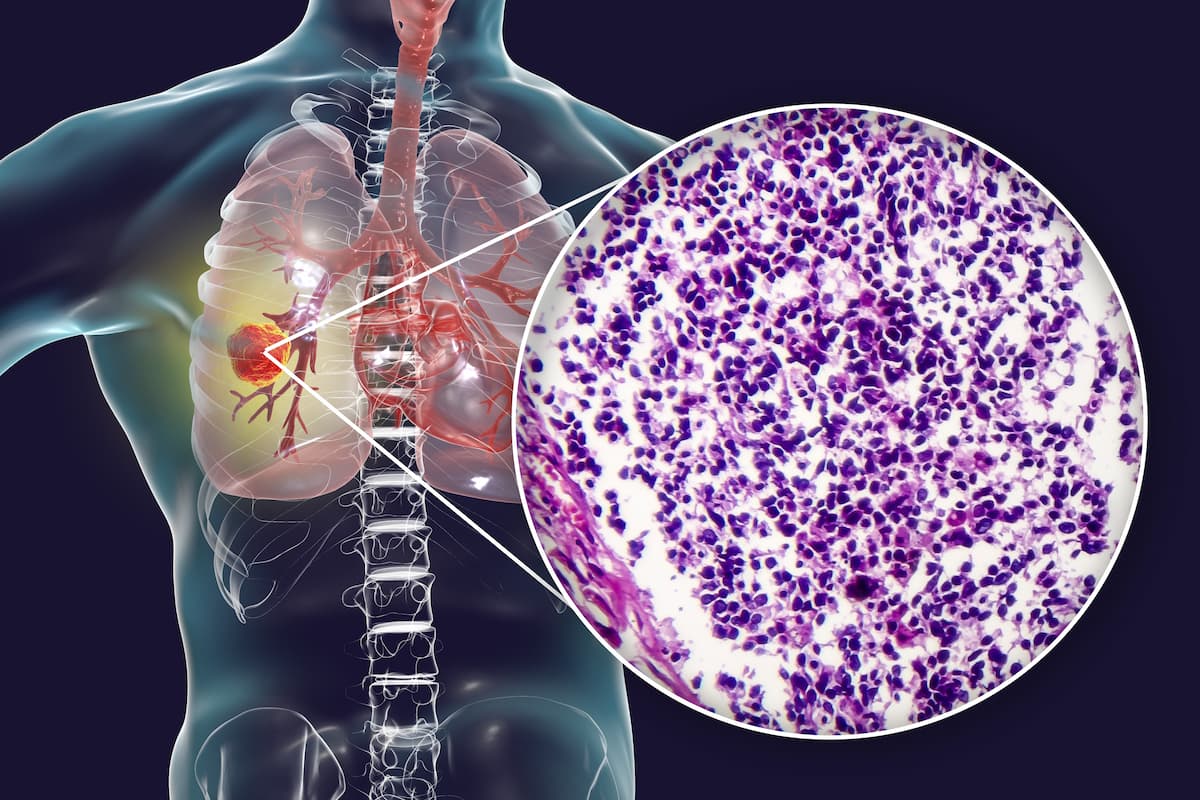
Non-Small Cell Lung Cancer (NSCLC)
Latest News


Durvalumab Plus Tremelimumab Fails to Meet Survival End Point in Phase 3 Metastatic NSCLC Study
Latest Videos

CME Content
More News

Results from the phase 2 ARC-7 trial found that zimberelimab, etrumadenant, and domvanalimab enhanced efficacy outcomes in patients with advanced non–small cell lung cancer.

The FDA has approved the Agilent Resolution ctDx FIRST assay for identifying patients with KRAS G12C–mutated non–small cell lung cancer who may benefit from adagrasib.

The FDA has approved an additional indication for pemetrexed injection plus pembrolizumab and chemotherapy in the treatment of metastatic non–small cell lung cancer without EGFR or ALK tumor aberrations.

Findings from the phase 3 CONTACT-01 trial indicated that cabozantinib and atezolizumab did not reach the primary end point of overall survival in patients with metastatic non–small cell lung cancer.

Alexander Spira, MD, PhD, FACP, of the Virginia Cancer Specialists, discusses how the FDA approval of adagrasib for KRAS G12C–mutated non–small cell lung cancer can provide benefit for this patient population.

Patients with KRAS G12C–mutated non–small cell lung cancer can now receive adagrasib therapy following the treatment’s recent accelerated approval by the FDA.

The EGFR inhibitor neratinib may be effective in the treatment of non-small cell lung cancer, according to updated interim findings from the phase 2 SUMMIT trial.
An expert from NYU Langone in New York City recently discussed the strengths and limitations of the oral tyrosine kinase inhibitor mobocertinib in platinum-pretreated patients with EGFR exon 20 insertion+ metastatic non-small cell lung cancer.

A complete response letter from the FDA indicated that poziotinib as a treatment for non-small cell lung cancer harboring HER2 exon 20 insertion mutations cannot be approved in its current form.
Erica C. Nakajima, MD, discussed a pooled analysis analyzing immune checkpoint inhibitors with or without chemotherapy in the frontline treatment of patients with KRAS-mutated non-small cell lung cancer and PD-L1 expression.

Pooling data with other radiation trials, looking more closely at central non-small cell lung cancer, and exploring secondary outcomes represent the next steps in terms of analyzing stereotactic body radiation (SBRT) vs conventional hypofractionated radiotherapy (CRT), according to Anand Swaminath, MD.

The National Institute for Health and Care Excellence has given a positive opinion of mobocertinib as a treatment for previously treated advanced non-small cell lung cancer with EGFR exon 20 insertion mutations.

Patients with unresectable stage III non-small cell lung cancer derived the most benefit from durvalumab when combined with either oleclumab or monalizumab vs durvalumab alone.

The FDA has approved tremelimumab plus durvalumab for patients with metastatic non–small cell lung cancer based on findings from arms 1 and 3 of the phase 3 POSEIDON trial.

Based on results of the phase 3 EMPOWER-Lung3 trial, frontline use of cemiplimab plus chemotherapy has been approved by the FDA for patients with advanced non–small cell lung cancer.

Results from the phase 1 ARROS-1 trial showed NVL-520 may stop tumor growth and yielded response in brain metastases in patients with ROS1-positive non–small cell lung cancer and other solid tumors.

A recent study found that giving sinoatrial node radiation therapy during chemoradiotherapy may increase the likelihood of atrial fibrillation in patients with small cell lung cancer and non–small cell lung cancer.

Expert oncologists review clinical scenarios to define best treatment practices for patients with EGFR-mutated non–small cell lung cancer.

Patients with KRAS G12C–mutant non–small cell lung cancer may benefit from BBP-398 plus sotorasib, which received fast track designation from the FDA.

Findings of the phase 2 PERLA trial indicated that dostarlimab combined with chemotherapy achieved promising responses in patients with metastatic non-squamous non-small cell lung cancer.

Patients with locally advanced or metastatic EGFR-mutant nonsquamous non–small cell lung cancer who progressed on an EGFR inhibitor may benefit from treatment with sintilimab plus bevacizumab biosimilar IBI305 and chemotherapy vs chemotherapy alone.

Fast track designation was granted to sapanisertib by the FDA for patients with unresectable or metastatic squamous cell non–small cell lung cancer who have an NRF2 mutation.

Based on results from a phase 3 trial, a bevacizumab biosimilar, bevacizumab-adcd, was approved by the FDA for patients with metastatic or recurrent non-squamous non–small cell lung in addition to 5 other disease types.

The Oncomine Dx Target Test was granted approval by the FDA as a companion diagnostic for RET fusion–positive thyroid cancer and RET fusion–positive non–small cell lung cancer.

The FDA’s Oncologic Drugs Advisory Committee voted against poziotinib as a treatment for patients with HER2 exon 20 insertion–mutated non–small cell lung cancer.