
Thyroid lesions identified in pediatric patients were often found to have one of the molecular abnormalities commonly seen in adults with thyroid lesions.

Your AI-Trained Oncology Knowledge Connection!


Thyroid lesions identified in pediatric patients were often found to have one of the molecular abnormalities commonly seen in adults with thyroid lesions.

A significant number of children who have completed treatment for acute lymphoblastic leukemia may be experiencing anxiety or depression, according to a new study.
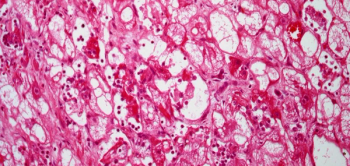
A 2-month-old child with a history of tuberous sclerosis presents with a cardiac lesion. After a biopsy is performed, what is your diagnosis?

Although a child’s socioeconomic status did not seem to significantly affect 5-year overall survival ALL, those children who were from high-poverty areas experienced early relapse compared with children who were from low-poverty areas.

There was no significant difference in the decline in IQ score over time between pediatric brain tumor patients treated with either photon radiation or proton beam radiation therapy, according to a new study.

Regular ultrasound surveillance of the thyroid revealed that about 7% of adult childhood cancer survivors who had radiation to the head or neck subsequently developed thyroid cancer.

Adult survivors of childhood astroglial tumors with significant vision loss are more likely to suffer various psychological and socioeconomic impacts such as unemployment.

Children with acute lymphocytic leukemia and their parents commonly over-report the amount of daily oral chemotherapy the child takes to treat the most common blood cancer in children.

Genetic variants increase the risk of osteonecrosis in children under age 10 with acute lymphoblastic leukemia.

Researchers have identified a genetic variant, 2R thymidylate synthase polymorphism, that is associated with an increased risk for avascular necrosis in children with ALL.

Targeted MAPK-pathway inhibitor therapies appear to have reduced tumor sizes in four children’s inoperable astrocytomas-tumors that had progressed despite chemotherapy.

PEG-asparaginase had both similar safety and efficacy to intramuscular native E coli l-asparaginase for the treatment of children with ALL in complete remission.

In this review, childhood cancer survivor populations at risk for metabolic late effects, as well as mechanisms and ongoing intervention studies, will be detailed.

It is too soon to determine the influence of radiation exposure on thyroid cancer risk among children and adolescents exposed to the 2011 Fukushima Daiichi nuclear power plant disaster in Japan, according to the lead author of findings presented at the 85th Annual Meeting of the ATA.

A new study has found an excess of thyroid cancer cases among children and adolescents who live near the Fukushima Daiichi nuclear power plant.

Risk for leukemia was significantly lower among children in Taiwan who were infected with the enterovirus compared with children who were not infected.

Even in the absence of cranial radiation therapy, survivors of childhood acute lymphoblastic leukemia (ALL) have decreased neurocognitive function years later.
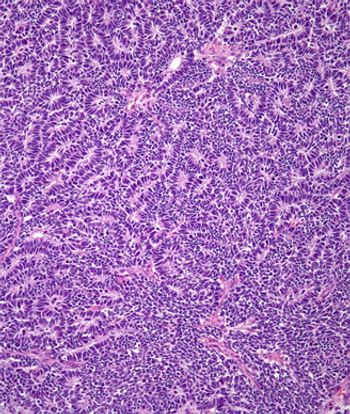
According to the AREN0532 and AREN0533 trials, adding additional drugs to a therapy regimen for Wilms tumor in children with high-risk disease improved outcomes.

A revised International Pediatric Non-Hodgkin Lymphoma Staging System (IPNHLSS) could allow for more precise staging for children and adolescents with NHL.

Stem cell transplantation from an HLA-genoidentical sibling or an HLA-matched unrelated donor did not affect outcomes among high-risk pediatric ALL patients.

The US Food and Drug Administration has approved dinutuximab (Unituxin) for the treatment of high-risk neuroblastoma in pediatric patients.
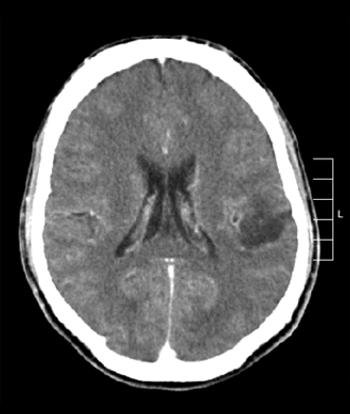
Researchers have identified two genetic characteristics that may distinguish pediatric secondary high-grade glioma from primary high-grade glioma.
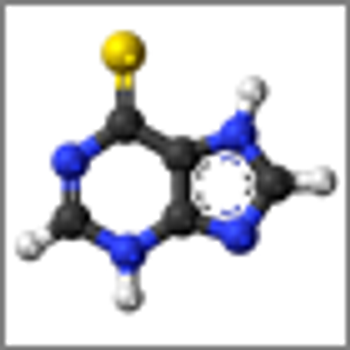
Using genome-wide association studies, researchers have identified a germline variant that is associated with intolerance to mercaptopurine in pediatric ALL.

In a recent study of pediatric ALL, minimal residual disease was able to predict patients who were at increased risk for relapse post-allogeneic stem cell transplantation.
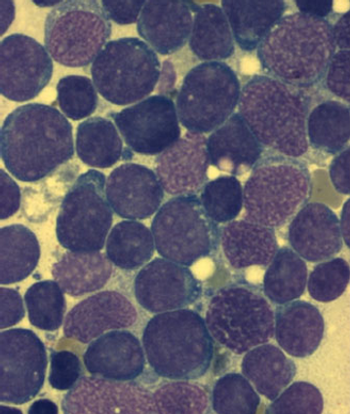
A large trial has shown that adolescents and young adults have better event-free and overall survival when treated on an intensive pediatric ALL regimen.