Use of the Cumulative Illness Rating Scale for Geriatrics may identify a greater comorbidity burden and a commensurate increase in risk of death among survivors of childhood cancer vs siblings and others.

Your AI-Trained Oncology Knowledge Connection!



Use of the Cumulative Illness Rating Scale for Geriatrics may identify a greater comorbidity burden and a commensurate increase in risk of death among survivors of childhood cancer vs siblings and others.
Investigators pause their evaluation of SC-DARIC33 in pediatric relapsed/refractory acute myeloid leukemia following a grade 5 serious adverse effect in the phase 1 PLAT-08 trial.

Adding blinatumomab to Interfant-06 chemotherapy appears to be feasible and safe in the treatment infants with acute lymphoblastic leukemia in a phase 2 trial.

Treatment with tovorafenib appears well tolerated among pediatric patients with low-grade glioma, according to findings from the phase 2 FIREFLY trial.
Patients under the age of 3 with neuroblastoma experienced no significant negative impact on survival when their disease was reclassified from high-risk to intermediate-risk and their therapy was thusly reduced.

A phase 2 trial indicates that limited surgery and post-operative proton therapy result in a high rate of tumor control and less severe complications in young patients with craniopharyngioma.

Investigators of a phase 1/2 trial report that CAR T cells targeting disialoganglioside GD2 may result in long-lasting antitumor activity in young patients with high-risk neuroblastoma.

Investigators highlight the importance of building partnerships between CAR T-cell centers and external hospitals to increase treatment referrals and access to clinical trials for patients with B-cell acute lymphoblastic leukemia.

Findings from 2 phase 3 trials support the recommendation to approve sodium thiosulfate to reduce the risk of cisplatin-related hearing loss in pediatric patients with solid tumors in Europe.

Findings from a cohort of the Childhood Cancer Survivor Study identify a prediction model that may accurately identify childhood cancer survivors at varying risks of late kidney failure.

Results from the phase 2 CDRB436G2201 trial lead to the approval of dabrafenib plus trametinib in patients with low-grade glioma and a BRAF V600E mutation who require systemic therapy.

Disparities in pediatric cancer survival in Europe might be mitigated through cross-border care, twinning programs, and use of pan-Europe cancer control plans.

The FDA initially issued a complete response letter for remestemcel-L in pediatric steroid-refractory acute graft-versus-host disease in October 2020, citing a need for an additional randomized, controlled trial to confirm the agent’s efficacy.
Findings from a retrospective cohort study suggest that circulating tumor DNA may be an important tool for risk-stratified treatment strategies in rhabdomyosarcoma.

Young adult survivors of childhood cancer may be at a higher risk of experiencing psychological and physical health issues associated with loneliness.

A medical oncologist and research audiologist from St. Jude Children’s Research Hospital discuss how sodium thiosulfate injection may improve quality of life by preventing cisplatin-associated ototoxicities in pediatric patients with localized non-metastatic tumors, although more research is needed.

A biologic license application that has been resubmitted to the FDA for remestemcel-L in pediatric graft-versus-host disease includes new long-term survival data from a phase 3 clinical trial.

The FDA gives orphan drug exclusivity to sodium phosphate injection for the prevention of cisplatin-related hearing loss in pediatric patients 1 month and older with localized, non-metastatic solid tumors.
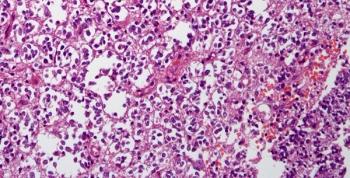
Data from the phase 2 FIREFLY-1 trial indicated that tovorafenib yielded responses among pretreated patients with recurrent or progressive pediatric low-grade glioma.

Adults and children with unresectable or metastatic alveolar soft part sarcoma—one of the rarest types of sarcoma—can now receive treatment with atezolizumab, which was given the nod of approval by the FDA.
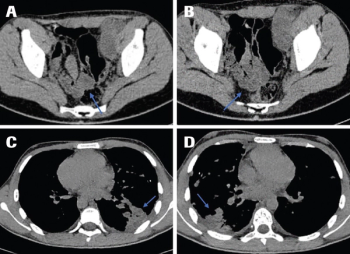
A systematic review of reported clinical cases and treatment strategies was performed to better understand the prognostic factors and to develop the best possible treatment option for a 16-year-old patient diagnosed with a malignant triton tumor in the lower extremity with distant metastases in the lungs.

Patients with previously untreated high-risk pediatric lymphoma can now receive treatment with brentuximab vedotin with the FDA’s nod of approval.

Findings from a phase 3 trial assessing nomacopan in patients with hematopoietic stem cell transplant–related thrombotic microangiopathy helped to support a rare pediatric designation from the FDA for the agent.

Members of the FDA’s Oncologic Drugs Advisory Committee indicated there isn’t enough evidence to definitively confirm the overall survival benefit of 131I-omburtamab in pediatric patients with central nervous system or leptomeningeal metastases stemming from neuroblastoma.
The FDA has given orphan drug designation to ET140203 ARTEMIS, an investigational therapy for hepatoblastoma, the safety and potential efficacy of which are being evaluated in the phase 1/2 ARYA-1 and ARYA-2 studies.