
The regulatory decision was supported by findings from the phase 1/2 BGB-11417-201 trial, which evaluated sonrotoclax monotherapy in those with MCL.

Your AI-Trained Oncology Knowledge Connection!


The regulatory decision was supported by findings from the phase 1/2 BGB-11417-201 trial, which evaluated sonrotoclax monotherapy in those with MCL.

Insurance and distance to a tertiary cancer center were 2 barriers to receiving high-quality breast cancer care, according to Rachel Greenup, MD, MPH.
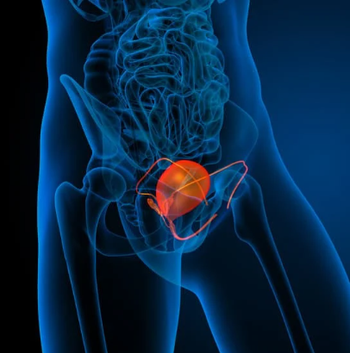
In the 177Lu plus SBRT arm, the median PFS was 17.6 months vs 7.4 months in the SBRT alone arm for patients with oligorecurrent HSPC.

Thomas Hope, MD, had not observed an adverse effect attributable to an infiltration across more than a decade of administering nuclear agents at UCSF.
Investigators are currently assessing the safety and preliminary activity of AVZO-103 among patients with advanced solid tumors in a phase 1/2 trial.

Results from MATTERHORN showed durvalumab plus FLOT improved EFS and OS compared with placebo plus FLOT in patients with gastric/GEJ cancers.

Results from the TRANSCEND NHL 001 trial showed that liso-cel achieved an ORR of 82.7% in patients with previously treated mantle cell lymphoma.

One patient with newly diagnosed glioblastoma multiforme reached 3 years of survival following treatment with temferon in a phase 1/2a trial.
PAS-004 was deemed safe and tolerable when given in 37 mg capsules, so the trial will proceed to administer the agent in 45 mg capsules.

When a patient may not have the capability of understanding or consenting to treatment options, Louis P. Voigt, MD; and Yesne Alici, MD, will utilize decision-making capacity techniques.
![According to John Henson, MD, “What we need are better treatments to control the [brain] tumor once it’s detected.”](https://cdn.sanity.io/images/0vv8moc6/cancernetwork/e0d29c38bb732429ae370e4ef7d1829a10c96446-2992x1684.png?w=350&fit=crop&auto=format)
According to John Henson, MD, “What we need are better treatments to control the [brain] tumor once it’s detected.”

According to John Henson, MD, patients with ovarian, breast, endometrial, and colon cancers benefit the most from proactive hereditary analyses.

Researchers have observed that HSC–derived innate lymphoid cells prevent GVHD by inducing interleukin 9 driven T-cell senescence.

Researchers have demonstrated that a tandem CAR T cell targeting mesothelin and MUC16 ectodomain is able to outmaneuver tumor heterogeneity, outperforming single target CAR T cells in mixed ovarian and pancreatic models.
Although both immune priming strategies numerically improved ORR and PFS vs olaparib monotherapy, the study was not powered for comparisons between arms.
No dose-limiting toxicities were observed among 12 patients with advanced EGFR-mutated NSCLC treated with quaratusugene ozeplasmid and osimertinib.

First-degree relatives of patients who passed away from pancreatic cancer should be genetically tested to identify their risk for the disease.

Daniel C. McFarland, DO, is joined by Louis P. Voigt, MD, and Yesne Alici, MD, who focused on decision-making capacity and patient-centered care.

Surgery and radiation chemotherapy can affect immunotherapy’s ability to target tumor cells in the nervous system, according to John Henson, MD.
Subgroup data from the ARASENS trial support darolutamide plus androgen deprivation therapy as a standard of care in metastatic HSPC regardless of age.
Treatment with MCS-8 yielded no serious adverse effects among patients at a high risk of developing prostate cancer.

Thinking about how to sequence additional agents following targeted therapy may be a key consideration in the future of lung cancer care.

Deeper short-term declines in quality of life occurred when combining cisplatin with radiation for those with intermediate-risk cervical cancer.
The blood-based test detected 31% of lung cancers one year prior to in-trial diagnosis compared with 8% of cancers identified by low-dose CT or Lung-RADS.

Fertility-sparing surgery showed comparable efficacy vs hysterectomy in early-stage cervical cancer, with a 5-year RFS rate of 92% vs 96.4%, respectively.

Endobronchial ultrasound, robotic bronchoscopy, or other expensive procedures may exacerbate financial toxicity for patients seeking lung cancer care.

Pharmacokinetic data from the phase 1/2 GO29781 study support the European approval of subcutaneous mosunetuzumab in this follicular lymphoma population.

Results from the KEYNOTE-905 trial led to the approval of pembrolizumab/enfortumab vedotin in muscle invasive bladder cancer.

Partial responses and stable disease were observed with PAS-004 in patients with advanced solid tumors harboring RAS, NF1, or RAF mutations.
