
Anne-Marie Jacob, a patient with multiple myeloma, discusses the treatment of multiple myeloma with bispecifics alongside a panel of experts.

Your AI-Trained Oncology Knowledge Connection!


Anne-Marie Jacob, a patient with multiple myeloma, discusses the treatment of multiple myeloma with bispecifics alongside a panel of experts.

Fifteen-year results of the ProtecT prostate cancer trial may support the findings of the study’s 10-year follow-up data, according to an expert from Dana-Farber Cancer Institute.

David L. DeRemer, PharmD, BCOP, and Bently P. Doonan, MD, MS, share a perspective on ocular toxicities associated with MEK inhibition.
A novel smoking cessation treatment for survivors of cervical cancer or cervical intraepithelial neoplasia appears to improve rates of abstinence in the short-term, but approximately matches the efficacy of standard treatment at 18 months.
The 10-year cumulative local recurrence rate in patients with early breast cancer receiving accelerated partial breast irradiation with multi-catheter brachytherapy does not significantly differ from those receiving whole-breast irradiation following breast-conserving surgery.
Data from the phase 3 TIGeR-PaC trial indicates that patients with locally advanced pancreatic cancer experienced notable survival benefit with RenovoGem vs chemotherapy.

Experts review the most recent data and discuss current strategies for the management of HR+ metastatic breast cancer.

Increasing age, higher Gleason scores, and higher pathologic stages are predictors of mortality in patients with prostate cancer, according to an expert from Dana-Farber Cancer Institute.

Clinical trials highlight benefits, including radiographic progression-free survival following treatment with radioligand 177Lu-PSMA-617 in pretreated patients with metastatic castration-resistant prostate cancer.
According to a retrospective analysis, bendamustine treatment correlates with a higher risk of infection and second malignancy compared with other treatments for indolent B-cell lymphoma.

ISB 1442, which received orphan drug designation from the FDA, is currently under investigation in a first-in-human phase 1/2 trial as a treatment for patients with relapsed/refractory multiple myeloma.

Joshua Richter, MD, discusses the optimal use of selinexor in the treatment of relapsed/refractory multiple myeloma.

Based on results from the phase 2 KEYNOTE-158, KEYNOTE-164, and KEYNOTE-051 trials, the FDA has given full approval to pembrolizumab for patients with microsatellite instability-high or mismatch repair deficient solid tumors.

Early data from ongoing clinical trials suggest the potential safety and efficacy of novel radium-223 combinations as treatment for metastatic castration-resistant prostate cancer.

Adding avelumab to VEGFR inhibitor axitinib may prolong survival and improve outcomes among patients with recurrent/metastatic adenoid cystic carcinoma.
Investigators are assessing tirabrutinib, which received orphan drug designation from the FDA, as a treatment for patients with relapsed or refractory primary central nervous system lymphoma in the phase 2 PROSPECT study.
Co-editor-in-Chief Howard S. Hochster, MD, highlights the most exciting developments from the 2023 Gastrointestinal Cancer Symposium hosted by the American Society of Clinical Oncology (ASCO).
Dostarlimab plus chemotherapy appears to improve progression-free survival vs placebo plus chemotherapy in patients with recurrent endometrial cancer in the phase 3 RUBY trial.

Santosh Rao, MD, discusses the formation of new guidelines informing the use of integrative oncology for patients with kidney cancer from the Society for Integrative Oncology.
Pembrolizumab plus chemotherapy followed by maintenance pembrolizumab reduced the risk of death or disease progression in patients with mismatch repair proficient or deficient advanced endometrial cancer.
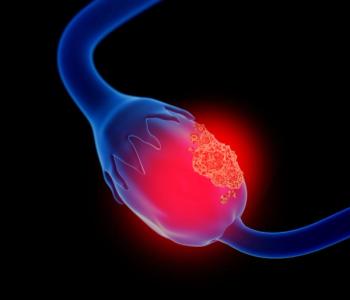
Patients with resistant or refractory ovarian cancer experience clinical benefits after receiving botensilimab plus balstilimab in the phase 1 C-800 study.

Toripalimab plus bevacizumab and platinum-based chemotherapy produces a “promising” response rate in those with metastatic cervical cancer, according to an expert from Peking Union Medical College.
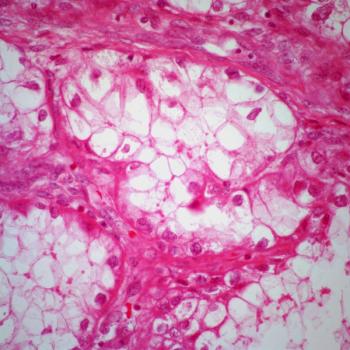
Adavosertib does not appear to be well tolerated in patients with previously treated recurrent or persistent uterine serous carcinoma in the phase 2b ADAGIO trial.
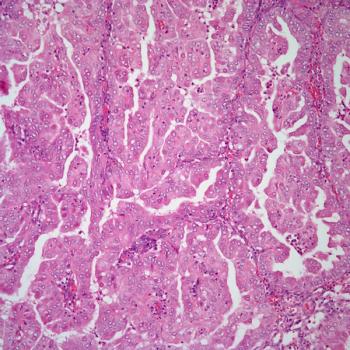
The phase 3 RUBY trial of dostarlimab plus carboplatin and paclitaxel significantly improved progression-free survival in patients with dMMR/MSI-H or MMRp/MSS endometrial cancer.
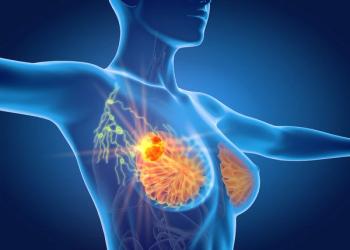
Overall survival after 10 years appears to be comparable regardless of whether patients 65 years or older received irradiation following surgery for low-risk, hormone receptor–positive breast cancer.
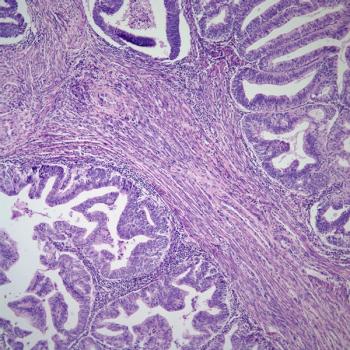
Durvalumab plus tremelimumab and hypofractionated radiotherapy yields observable clinical benefits in patients with gynecologic cancers, according to an expert from The University of Texas MD Anderson Cancer Center.

Niraparib maintenance therapy for recurrent ovarian cancer does not yield a significant overall survival benefit in an updated analysis of the phase 3 ENGOT-OV16/NOVA study.

An expert from Dana-Farber Cancer Institute discusses findings from the final overall survival analysis of the phase 3 ENGOT-OV16/NOVA trial.
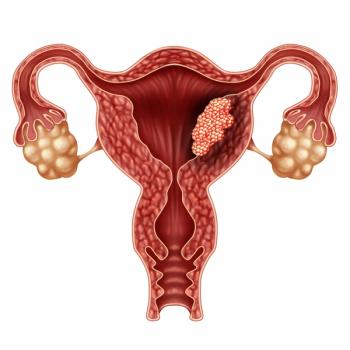
Patients with advanced endometrial cancer experience early responses to lenvatinib plus pembrolizumab in both patients who were mismatch repair proficient and all-comers.
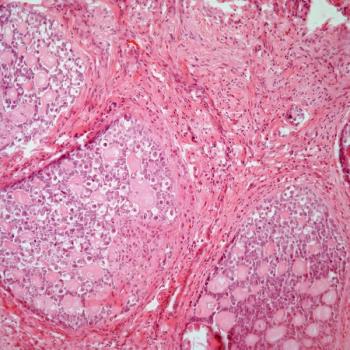
The Carolina Frailty Index Score indicates that patients with ovarian cancer classified as frail are highly more likely to die than patients classified as robust.