Preliminary findings indicate that P-PSMA-101, a CAR T-cell therapy, may be efficacious in patient with metastatic castration-resistant prostate cancer.

Your AI-Trained Oncology Knowledge Connection!


Preliminary findings indicate that P-PSMA-101, a CAR T-cell therapy, may be efficacious in patient with metastatic castration-resistant prostate cancer.
Pediatric patients with low-risk acute lymphoblastic leukemia may be able to omit treatment with vincristine and dexamethasone pulse therapy after the first year of treatment.

Autologous hematopoietic stem-cell transplantation appears to improved survival compared with interferon alfa maintenance therapy for patients with previously untreated mantle cell lymphoma.
A number of prognostic and treatment related factors have been found to impact health-related quality of life among survivors of breast cancer.
Patients with cancer and those without cancer had similar serologic results 6-months after receiving their second BNT162b2 vaccine.
Patients with relapsed or refractory multiple myeloma with or without t(11;14) experienced promising responses after being treated with venetoclax plus daratumumab and dexamethasone, as well as venetoclax plus daratumumab, dexamethasone, and bortezomib.
The risk of infection during induction chemotherapy decreased among patients with acute myeloid leukemia treated with romyelocel-L.
Data from patients with gastric or gastroesophageal junction cancers who were treated with nivolumab showed that effects in the genomic pathways of the gut microbiome may be significantly associated with survival.

Zanubrutinib has been approved by the FDA for the treatment of patients with Waldenströms macroglobulinemia based on the results of the phase 3 ASPEN trial.

High intensity interval training yielded decreased prostate-specific antigen levels and velocity, as well as lowering prostate cell growth in men with localized prostate cancer.
The combination of standard chemoradiotherapy plus avelumab demonstrated clinical activity while maintaining a tolerable safety profile for patients with locally advanced rectal cancer.
New diagnoses of common cancer types declined significantly during the first year of the COVID-19 pandemic.
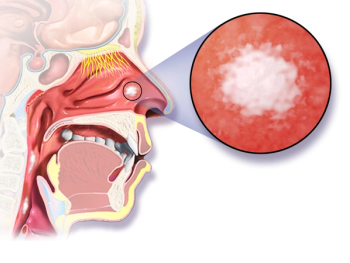
Patients with advanced nasopharyngeal carcinoma and who were treated with gemcitabine plus cisplatin experienced a longer overall survival and improved progression-free survival vs fluorouracil plus cisplatin.

Latina breast cancer survivors are less likely to seek counseling due to concerns that the counselor will not understand their values or have linguistic challenges.

The FDA has granted a full approval to pembrolizumab for the treatment of platinum-ineligible patients with urothelial cancer.

Compared with anti-thymocyte globulin, post-transplant cyclophosphamide appears to more successfully prevent graft-versus-host disease in patients with acute lymphoblastic leukemia, resulting in a lower incidence of relapse and improved survival.

Low study quality was determined in an analysis of real-world studies across multiple drug indications in cancer.

Survivorship incorporated into primary cancer care may be more cost effective and lead to better clinical and patient-reported outcomes in patients with breast cancer and other malignancies.
A new set of guidelines for utilizing radiation therapy in adult patients with soft tissue sarcoma has been published by the American Society for Radiation Oncology.
Patients with relapsed or regractory B-cell malignancies who were treated with zanubrutinib and zandelisib demonstrated high response rates across several treatment cohorts.
Patients with non–small cell lung cancer and small-cell lung cancer with bone metastases may benefit from extended dosing with zoledronic acid.
Minority patients with aggressive B-cell lymphoma experienced equitable outcomes through more accessible care and the use of the nurse navigators.
Updates to the National Comprehensive Cancer Network guidelines on the COVID-19 vaccine for patients with cancer indicate that this group should be prioritized for a third dose.

Patients with high-risk early breast cancer who received zoledronate for over 2 years did not experience an improvement in prognosis.

Gilles Salles, MD, PhD, and Kami Maddocks, MD, discuss relapsed/refractory diffuse large B-cell lymphoma therapeutic options and important data from the L-MIND trial.

The use of hypofractionated image-guide radiotherapy was not found to improve overall survival for patients with non–small cell lung cancer and poor performance status versus conventional fractionated radiation techniques.

This special episode of “Oncology Peer Review On-The-Go” includes a discussion on relapsed/refractory follicular lymphoma with Javier Munoz, MD, MS, FACP.

Responses to a cross-sectional survey brought to light disparities through important aspects of diagnosing and treating brain metastases.
CancerNetwork® spoke with Quita Highsmith and Monica Baskin, MD, about the Advancing Inclusive Research Alliance and efforts to include more diverse populations in clinical trials.

Atezolizumab will no longer be available for the treatment of patients with PD-L1–positive triple-negative breast cancer following withdrawal of the indication by the agent’s developer.