
Data from a phase 2 clinical trial with pembrolizumab and cetuximab yielded promising clinical activity in patients with recurrent or metastatic head and neck squamous cell carcinoma.

Your AI-Trained Oncology Knowledge Connection!


Data from a phase 2 clinical trial with pembrolizumab and cetuximab yielded promising clinical activity in patients with recurrent or metastatic head and neck squamous cell carcinoma.
Novel eganelisib shows survival-extending potential in patients with triple-negative breast and bladder cancers.

Julie Vose, MD, MBA, a professor of internal medicine in the Division of Oncology and Hematology at the University of Nebraska Medical Center discussed the benefits of CAR T-cell for patients with follicular lymphoma.
The phase 2 clinical trial is currently recruiting patients with pediatric low-grade glioma to be treated with DAY101 following a rare pediatric disease designation by the FDA.

Oncologists are trying to remedy the proposed cuts to radiation oncology facilities from the Centers for Medicare and Medicaid Services to lessen health disparities and improve equitable access to care.
A phase 1/2 trial featuring lanraplenib/gilteritinib in patients with FLT3-mutant acute myeloid leukemia will proceed following the FDA’s clearance of an investigational new drug application.
Patients with PD-1 inhibitor-resistant melanoma who were treated with ipilimumab plus anti–PD-1 therapy saw significantly better long-term responses than those on ipilimumab alone.

Based on results of the KEYNOTE-522 trial, the FDA approved pembrolizumab, the first immunotherapy for this indication, plus chemotherapy as neoadjuvant treatment for patients with early-stage triple-negative breast cancer.

CancerNetwork® sat down with Julie Vose, MD, MBA, at the 2021 American Society of Clinical Oncology Annual Meeting to talk about a clinical trial comparing acalabrutinib versus ibrutinib for patients with chronic lymphocytic leukemia.

Here are some of the important updates from CancerNetwork last week you might have missed in the world of oncology, featuring novel combinations for triple-negative breast cancer, genetic testing for gastrointestinal malignancies, and the FDA approval of belumosudil for chronic graft-versus-host disease.
Recent data indicated that the combination of neoadjuvant docetaxel, oxaliplatin, and S-1 followed by surgery and adjuvant S-1 was effective and tolerable in a population of Korean patients who had locally advanced gastric cancer.
Patients with cancer who are Black or live in low-income areas were found to be at a higher risk of COVID-19 complications, according to recent findings.
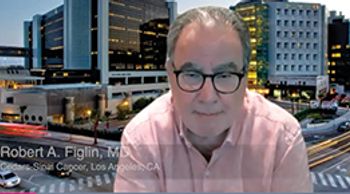
Robert A. Figlin, MD, discusses the potential role of adjuvant immunotherapy for patients with bladder cancer.
Bempegaldesleukin plus nivolumab demonstrated positive antitumor activity while maintaining a tolerable safety profile in the PIVOT-02 trial for patients with previously untreated metastatic melanoma in the first-line setting.

CancerNetwork® sat down with Jonathan Spicer, MD, PhD, at the 2021 American Society of Clinical Oncology Annual Meeting to talk about how communication across a multidisciplinary team was necessary to the success of neoadjuvant immunotherapy administration in CheckMate 816.

A cross-sectional study from patient survey indicates knowledge of palliative care services are lacking across patient populations.

At ASCO 2021, CancerNetwork® spoke with Stephen Liu, MD, about his research on the ARROW trial and how these results might impact the treatment of patients with RET fusion–positive non–small cell lung cancer who are potential candidates for treatment with pralsetinib.
For patients with large B-cell lymphoma who have undergone treatment with axicabtagene ciloleucel, ctDNA monitoring may help to improve early detection of recurrent disease.
CancerNetwork® spoke with Jeffery Auletta, MD, about how the National Marrow Donor Program/Be The Match is using research initiatives to expand eligibility for stem cell transplants in for patient with acute leukemias and myelodysplastic syndrome.
Results of a phase 2 trial show that ibrutinib was capable of inducing responses in some patients with hairy cell leukemia who were previously treated with standard therapy options in a prior line.
Patients with unresectable hepatitis B virus–positive hepatocellular carcinoma have achieved significantly better overall survival and progression-free survival following treatment with sintilimab plus IBI305 vs sorafenib.

CancerNetwork® sat down with John Heymach, MD, PhD, at the 2021 American Society of Clinical Oncology Annual Meeting to talk adjuvant immunotherapy for recurrence-free survival in resected non–small cell lung cancer.
Patients with hematologic malignancies and COVID-19 may experience a survival benefit after receiving convalescent plasma therapy.
Patients with relapsed/refractory mantle cell lymphoma experienced promising response rates after undergoing treatment with zanubrutinib.
Personalized risk assessments that utilize pathogenic variations can help detect CRC early in patients with Lynch Syndrome.

Phase 3 results demonstrating the superiority of the pembrolizumab plus lenvatinib combination vs standard chemotherapy served as a confirmatory trial for its approval in certain patients with advanced endometrial cancer.

Cynthia Ma, MD, PhD, explains key efficacy findings regarding HER-targeted therapy to treat patients with HER2-mutant breast cancer.

Sintilimab with gemcitabine plus platinum produced superior clinical benefit for patients with locally advanced or metastatic squamous cell non–small cell lung cancer when compared with gemcitabine plus platinum alone.
An oncology pharmacist talks secondary malignancies noted with tazemetostat in the treatment of follicular lymphoma.
Compared with a control therapy, ruxolitinib increased overall response and improved failure-free survival for patients with glucocorticoid-refractory or -dependent chronic graft-versus-host disease.