
Jane L. Meisel, MD
Articles by Jane L. Meisel, MD


Panelists discuss evolving frontline treatment strategies and future directions in HER2+ breast cancer, focusing on the latest advancements and emerging approaches in managing this subtype.

Panelists discuss a clinical scenario involving tucatinib-based regimens in the management of brain metastases in the second-line metastatic setting, exploring treatment options and clinical decision-making.

Panelists discuss intracranial efficacy data with tucatinib and T-DXd in the treatment of brain metastases, highlighting key findings and their implications for clinical practice.

Panelists discuss the strategic screening and management of brain metastases in HER2+ breast cancer, focusing on current approaches and emerging strategies to address this complication.

Panelists discuss how a third-line approach for a 54-year-old patient treated with a tucatinib-based regimen involves careful consideration of prior treatment responses, comorbidities, and the potential benefits of combining tucatinib with capecitabine and trastuzumab to improve outcomes in HER2+ metastatic breast cancer.

Panelists discuss how a third-line approach for a 54-year-old patient treated with a tucatinib-based regimen involves careful consideration of prior treatment responses, comorbidities, and the potential benefits of combining tucatinib with capecitabine and trastuzumab to improve outcomes in HER2+ metastatic breast cancer.

Panelists discuss how managing trastuzumab deruxtecan (T-DXd)–related toxicity and intolerance in later-line HER2+ breast cancer requires close monitoring, early detection of adverse effects like interstitial lung disease, and strategic adjustments to treatment regimens for improved patient safety and outcomes.

Panelists discuss how clinical data and treatment selection in later-line HER2+ metastatic breast cancer are informed by the latest therapeutic advancements, including targeted therapies like trastuzumab deruxtecan (T-DXd) and tucatinib, to optimize patient outcomes and manage progression.

Experts from Emory Winship Cancer Institute discuss how the FDA approval of sacituzumab govitecan will impact the treatment of breast cancer.
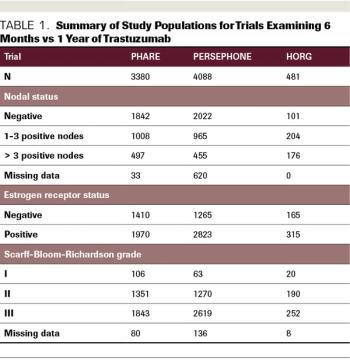
ABSTRACT Prior to the introduction of trastuzumab, the first targeted anti-HER2 agent, in 1998, patients diagnosed with HER2-positive breast cancer felt like they were being handed a death sentence. Despite treatment with aggressive chemotherapy, their tumors recurred faster, more often spread to brain and liver, and were associated with higher rates of death than HER2-negative tumors. However, in the 1980s, cancer researchers and oncologists recognized that HER2 could be targeted by a small molecule that binds to the receptor on the cell surface and blocks the signal telling the cell to divide. This small molecule was called trastuzumab, and it eventually completely changed how HER2-positive breast cancer was treated. The drug was first approved in the metastatic setting, and then the results of 2 pivotal randomized control trials demonstrated that the administration of trastuzumab in the adjuvant setting decreased the risk of breast cancer recurrence by 50%. These trials showed trastuzumab to be unequivocally effective in the adjuvant setting and the HERA trial results led to the adoption of 1 year of adjuvant trastuzumab as the standard of care. Since that time, the field of anti–HER2-targeted therapy has exploded, with the development of multiple targeted agents for use in the advanced and up-front settings. Although trastuzumab significantly improves outcomes for women diagnosed with HER2-positive breast cancer and has few adverse effects (AEs), the disadvantages are that it requires intravenous administration every 3 weeks and can be associated with cardiac AEs. It is also expensive. Given all of these factors, the question of whether a duration of trastuzumab that is shorter than 1 year may be acceptable for some patients with early-stage HER2-positive breast cancer is an important and very relevant one. Here, we will review the studies that have examined this question and evaluate their results.

Here we review current guidelines on breast and ovarian cancer screening, prophylactic surgery, and other risk-reduction strategies in patients with these mutations, and we detail the data that drive these recommendations.
Latest Updated Articles
 Optimizing the Duration of Trastuzumab: A Fresh Perspective
Optimizing the Duration of Trastuzumab: A Fresh PerspectivePublished: August 12th 2020 | Updated:
 Overview of Clinical Data and Treatment Selection in Later-Line HER2+ Metastatic Breast Cancer
Overview of Clinical Data and Treatment Selection in Later-Line HER2+ Metastatic Breast CancerPublished: December 23rd 2024 | Updated:
 Prevention and Screening in Hereditary Breast and Ovarian Cancer
Prevention and Screening in Hereditary Breast and Ovarian CancerPublished: October 16th 2016 | Updated:
 Oncology On-the-Go Podcast: Sacituzumab Govitecan for HR+/HER2– Advanced Breast Cancer
Oncology On-the-Go Podcast: Sacituzumab Govitecan for HR+/HER2– Advanced Breast CancerPublished: February 20th 2023 | Updated:
