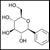
The review by Rampersaud and colleagues provides an excellent summary of the scientific rationale for using hyperthermia to treat cancer and of the current status of combinations of hyperthermia and chemotherapy or radiotherapy. In view of the demonstrated efficacy of the combination of intravescial hyperthermia and mitomycin C (MMC) therapy in preventing the progression and recurrence of non–muscle-invading bladder cancer (NMIBC) in several clinical trials, Rampersaud and colleagues advocate additional studies to further optimize the delivery of hyperthermia and to delineate its clinical utility in this disease.