Gynecologic Cancers
Latest News
Latest Videos

CME Content
More News

A prospective trial may help affirm ctDNA as a non-invasive option of predicting responses to radiotherapy among those with gynecologic cancers.

ctDNA reductions or clearance also appeared to correlate with a decrease in disease burden during the pre-boost phase of radiotherapy.

Investigators evaluated ctDNA as a potentially noninvasive method to predict response to radiotherapy among those with gynecologic malignancies.

Ginger J. Gardner, MD, FACOG, walked through the strides of gynecologic cancer research and emphasized the efforts that still need to be accomplished.

The Foundation for Women’s Cancer provides multicultural resources for patients with gynecologic cancers to help address gaps in care.

Ginger J. Gardner, MD, FACOG, addresses the growing uterine cancer cases among patients in the United States and the need for greater genetic testing.

Ginger J. Gardner, MD, FACOG, discussed the state of gynecologic cancers and her role in empowering research, education, and awareness surrounding them.

A new surgical option of uterine transposition may help preserve fertility for women who have cancers of the pelvic region.

Uterine transposition is a newer tactic in the surgical oncology field to help preserve fertility for patients undergoing pelvic radiation.

As the uterus is moved to the anterior abdominal wall, patients now have a chance of preserving their fertility during radiation therapy.

Pluta Cancer Center clinicians discussed how to improve sexual health outcomes for patients diagnosed with gynecological cancer.
PAX8, a marker for aggressive disease in numerous cancer types, was more highly expressed in Black patients with uterine serous carcinoma.

Data support subcutaneous envafolimab plus lenvatinib as a promising new therapy option in advanced endometrial cancer.

The CARACO study shows that adding retroperitoneal lymphadenectomy to cytoreductive surgery did not improve survival in advanced ovarian cancer.
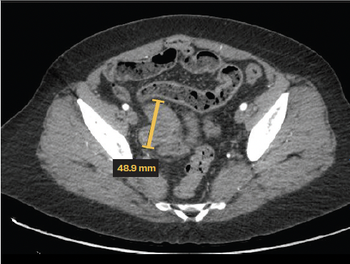
Learn more about a 56-year-old woman diagnosed with well-differentiated papillary mesothelioma, and how she was diagnosed and properly treated.
Approval of the self-collection solution may reduce barriers to sample collection and increase access to cervical cancer screening.

The CheckMate 358 trial assessed various doses of nivolumab with or without ipilimumab for recurrent or metastatic cervical cancer.
Developers will work with investigators of the phase 3 KEYNOTE-B21 trial to share their findings with the scientific community.

The FDA has set a Prescription Drug User Fee Act date of August 23, 2024, for dostarlimab in all types of primary advanced endometrial cancer.

Combining rintatolimod with pembrolizumab may confer a synergistic effect in patients with recurrent ovarian cancer.
Even when adjusting for prior taxane responses in patients with ovarian cancer, ixabepilone/bevacizumab appears to yield survival benefits in a phase 2 trial.

Findings from the phase 2 RAMP 201 trial highlight responses with avutometinib/defactinib in those with KRAS-mutated low-grade serous ovarian cancer and other patient subgroups.

Nab-Sirolimus appears to produce responses in patients with perivascular epithelioid sarcoma regardless of TSC1/TSC2 mutation status.

Ex vivo generation of CD3-positive, CD56-positive Natural Killer-Like (NKT) with CRX100 was successful in all 7 patients with ovarian cancer in a phase 1 trial.

Data from the DUO-E trial support potential new durvalumab-based treatment options for patients with advanced or recurrent endometrial cancer.