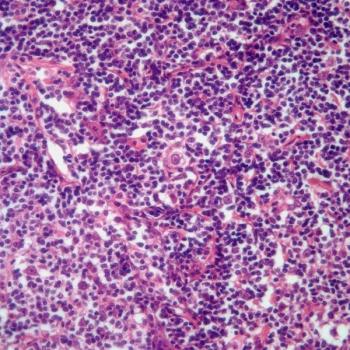
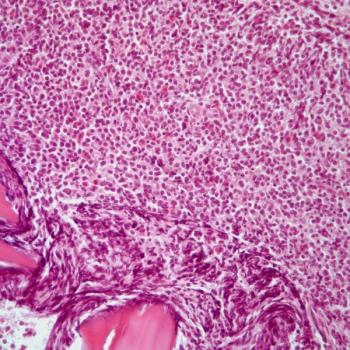
A 22-year-old man presents with enlarged axillary lymph nodes. What is your diagnosis?

Your AI-Trained Oncology Knowledge Connection!


A 22-year-old man presents with enlarged axillary lymph nodes. What is your diagnosis?

Stem cell transplantation from an HLA-genoidentical sibling or an HLA-matched unrelated donor did not affect outcomes among high-risk pediatric ALL patients.
A single-arm, open-label trial in Australia found that selective early switching from imatinib to nilotinib is feasible and effective in patients with CML.

CML patients with high CIP2A levels treated with second-generation tyrosine kinase inhibitors have better outcomes than those treated with imatinib.
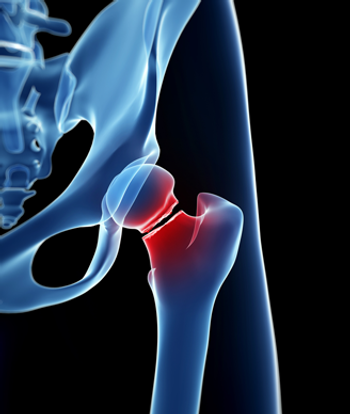
Results of a single-institution study found the risk for fracture among survivors of hematopoietic stem-cell transplantation increased by nearly eight times.

The next few years hold great promise, as new agents emerge and others consolidate their place on our shelves. We will be forced to rethink strategies and redefine management as a new era of immuno-oncology dawns.

Here we review monoclonal antibodies that have received FDA approval for the treatment of NHL and CLL in the last 5 years, as well as promising agents in development.
In a phase I trial, DT2219, the novel bispecific ligand-directed toxin, has shown activity against relapsed and refractory B-cell lymphoma and leukemia.
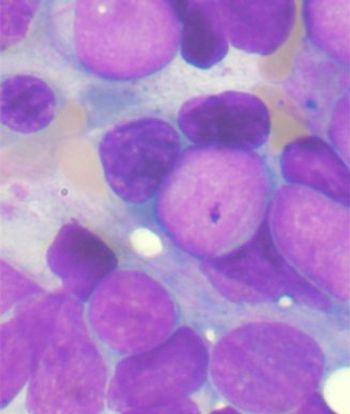
A 32-year-old man presents with malaise and fatigue. A bone marrow biopsy is performed. What is your diagnosis?
Treatment with carfilzomib and dexamethasone doubled the PFS in relapsed multiple myeloma patients compared with treatment with bortezomib and dexamethasone.
In patients with AML, post-therapy parameters including minimal residual disease and remission were found to be independent prognostic factors for outcomes.
Induction treatment for acute myeloid leukemia with amonafide L-malate/cytarabine failed to improve the rate of complete response over daunorubicin/cytarabine.
Multiple myeloma patients had better overall survival if they had prior knowledge of having monoclonal gammopathy of undetermined significance.
Researchers have identified patient factors linked with the discontinuation of ibrutinib therapy for reasons other than disease progression.

The FDA has approved panobinostat (Farydak), in combination with bortezomib and dexamethasone, for treating patients with multiple myeloma.
New data indicates that the order in which people acquired certain somatic mutations influenced several clinical features of myeloproliferative neoplasms.

The FDA has expanded the use of oral lenalidomide in multiple myeloma to include its use in combination with dexamethasone for newly diagnosed patients.
A new study suggests that cancer patients do not often request unnecessary and sometimes costly tests or treatments.

A new study finds that childhood cancer survivors are at risk for pituitary hormone deficiencies after radiotherapy treatment to the head.

Despite an association with improved survival, elderly DLBCL patients are less likely to receive standard-of-care chemotherapy with R-CHOP.
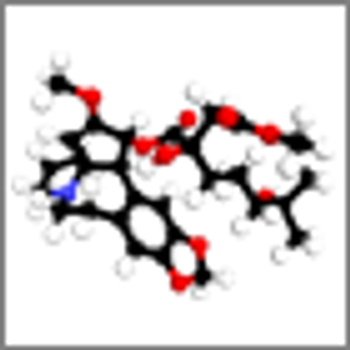
In two phase II trials, the protein synthesis inhibitor omacetaxine offered long-term efficacy in some patients with chronic-phase and accelerated-phase CML.
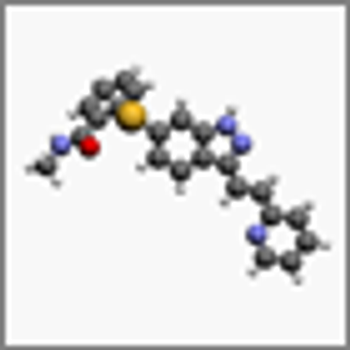
Researchers have shown that axitinib could be repurposed as a treatment for CML patients resistant to standard TKIs through a certain molecular mechanism.

A scoring system that measured frailty in elderly patients with multiple myeloma was able to accurately predict mortality and risk of toxicity.

The FDA has expanded the approved use of ibrutinib (Imbruvica) to include patients with Waldenström macroglobulinemia, a rare type of non-Hodgkin lymphoma.
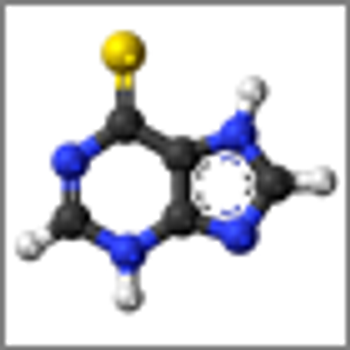
Using genome-wide association studies, researchers have identified a germline variant that is associated with intolerance to mercaptopurine in pediatric ALL.