Patients who experienced early relapse of multiple myeloma after undergoing autologous stem cell transplantation had worse progression-free and overall survival.

Your AI-Trained Oncology Knowledge Connection!


Patients who experienced early relapse of multiple myeloma after undergoing autologous stem cell transplantation had worse progression-free and overall survival.

Survivors of childhood Hodgkin lymphoma who went on to regularly complete vigorous exercise had a lower risk of cardiovascular events later in life.

Ninety percent of patients with relapsed/refractory acute lymphoblastic leukemia achieved complete remission after a T-cell therapy treatment targeting CD19.
Monitoring minimal residual disease and real-time quantitative polymerase chain reaction can predict relapse in pediatric acute lymphoblastic leukemia patients.

A phase III trial of vosaroxin failed to meet its primary overall survival endpoint in patients with first relapsed or refractory acute myeloid leukemia.

Myeloma patients treated with melphalan prior to transplant had reductions in chemo-induced nausea/vomiting when given granisetron/dexamethasone and aprepitant.

In this interview we discuss the use of newly approved agents for chronic lymphocytic leukemia, next-generation therapies in the pipeline, and how the treatment of this type of leukemia has changed.
A regimen of gemcitabine/dexamethasone/cisplatin was as effective and less toxic than the current standard of care for treating relapsed, refractory lymphoma.
Panobinostat added to bortezomib/dexamethasone for treating relapsed/refractory multiple myeloma improved progression-free survival and complete response rates.
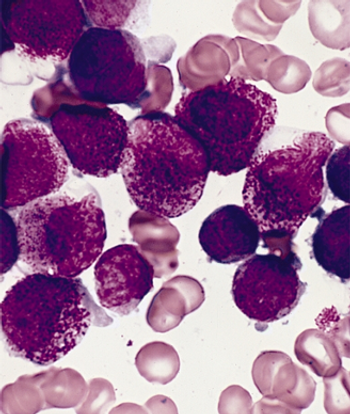
Patients with non-high-risk APL can achieve better clinical outcomes when treated with ATRA plus arsenic trioxide compared with ATRA plus chemotherapy, a new study found.
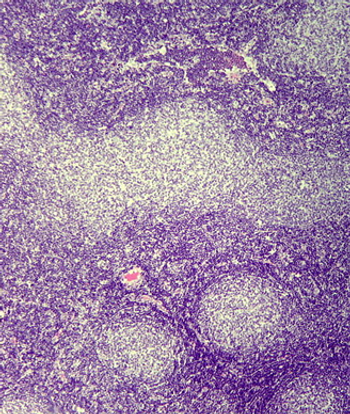
Data from a pooled analysis show that PET-CT provided better response assessment than did CT and predicted inferior survival in follicular lymphoma patients.

Hodgkin lymphoma survivors who were treated with infradiaphragmatic radiotherapy have an increased risk of developing diabetes mellitus, says a new study.

A study of more than 4,500 patients with acute myeloid leukemia (AML) found that predicting resistance to therapy remains an elusive practice.
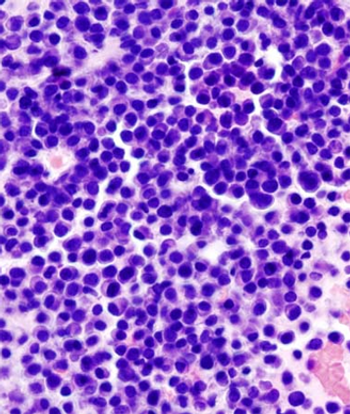
Multiple myeloma patients may already suffer from sensory deficits prior to treatment, likely due to disease-related decreases in peripheral innervation density.

The rationale for maintenance therapy in indolent non-Hodgkin lymphoma was derived from historical data suggesting that despite robust response rates to standard therapy, most patients eventually relapse and disease-free intervals become progressively shorter.

While definitions of follicular lymphoma maintenance therapy in clinical trials and clinical practice have been somewhat variable, ideally maintenance therapy would be limited to patients in complete remission or with minimal residual disease following initial therapy
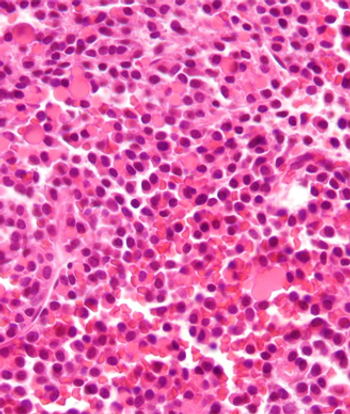
The optimal treatment strategy for newly diagnosed multiple myeloma is consolidation with melphalan, stem-cell transplantation, then lenalidomide maintenance.

STAT3 inhibition using a novel compound restored sensitivity to TKIs in CML cells that had shown resistance independent of BCR-ABL1 kinase activity.

Continuous treatment with lenalidomide plus dexamethasone improved progression-free and overall survival in transplant-ineligible multiple myeloma patients.
A laboratory study found that natural killer cells could be multiplied from the blood of patients to fight off precursor B-lineage acute lymphoblastic leukemia.
Survivors of Hodgkin lymphoma may be at increased risk of diabetes if they were exposed to radiation to the para-aortic lymph nodes and spleen during treatment.
A new study confirms that patients with primary cutaneous diffuse large B-cell lymphoma who have a MYD88 mutation have a shorter disease-specific survival.
Phase II study results show that a new combination of drugs known as R2CHOP had promising efficacy in the treatment of relapsed diffuse large B-cell lymphoma.
The addition of gemtuzumab-ozogamicin to standard chemo improved event-free survival in children and young adults with newly diagnosed acute myeloid leukemia.
Treatment with bortezomib prior to autologous stem cell transplant resulted in superior outcomes in multiple myeloma patients with end-stage renal failure.