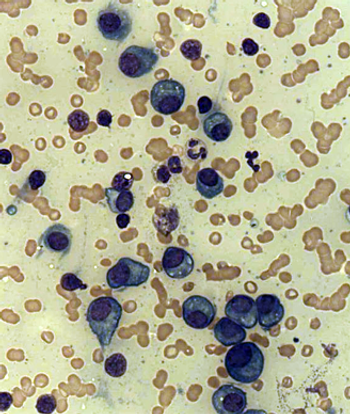
Two alternative multiple myeloma plasma cell surface markers have been identified and could be important for subclassification, prognostication, and treatment stratification of patients with multiple myeloma.

Your AI-Trained Oncology Knowledge Connection!


Two alternative multiple myeloma plasma cell surface markers have been identified and could be important for subclassification, prognostication, and treatment stratification of patients with multiple myeloma.
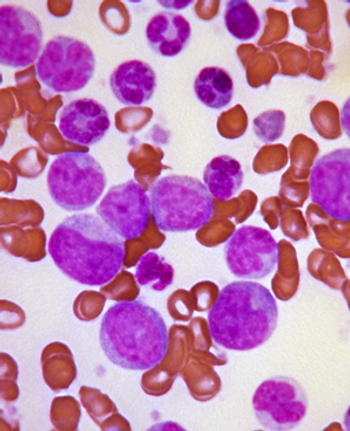
A deep molecular response to imatinib, achieved by most chronic myeloid leukemia patients who receive the drug, is predictive of better overall survival, according to a new study.
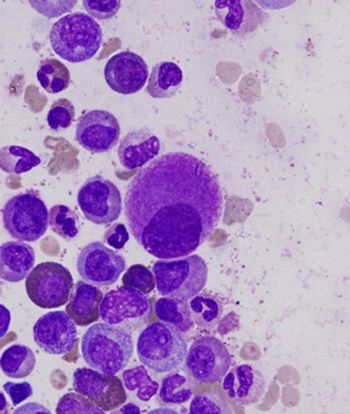
Modulation of the bone marrow microenvironment with parathyroid hormone may be a feasible way to dramatically reduce counts of leukemia stem cells in chronic myeloid leukemia patients, according to new research in mice.
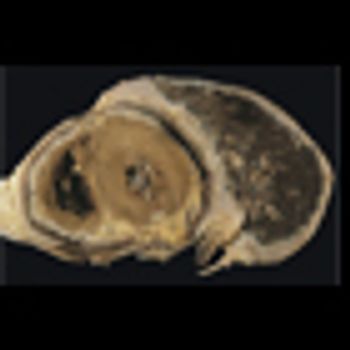
Mesotheliomas that arise after patients receive radiation therapy for lymphoma have unusual histologic features, and those patients tend to be younger and tend to survive longer than more common asbestos-related mesothelioma patients.
A new phase II trial found that a regimen containing rituximab, gemcitabine, cyclophosphamide, vincristine, and prednisolone is active and reasonably safe in patients with diffuse large B-cell lymphoma and coexisting cardiac disease.
A study aimed at defining the natural history of breast implant-associated anaplastic large-cell lymphoma found that outcomes differ between those cases where the disease is confined within the fibrous capsule surrounding the implant and those where a mass is present in the breast.

Although the prospect is tempting, we do not believe there are sufficient grounds at this time to abandon bone marrow biopsy in patients with lymphoma. It still provides robust prognostic information, and in the majority of patients it remains an indispensable staging tool.

Clearly, eliminating a bone marrow biopsy in appropriate patients would be another step in the direction of minimizing the torture to which they are subjected.

Because of challenges in making the correct diagnosis and the physician’s reluctance to administer chemotherapy for a disease characterized by such a low tumoral mass, patients may experience a delay in the initiation of appropriate treatment.

While the name POEMS (polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes) provides a nice acronym for a collection of seemingly disparate features, the diagnosis does not require that all these elements be present, and many other features are not included.
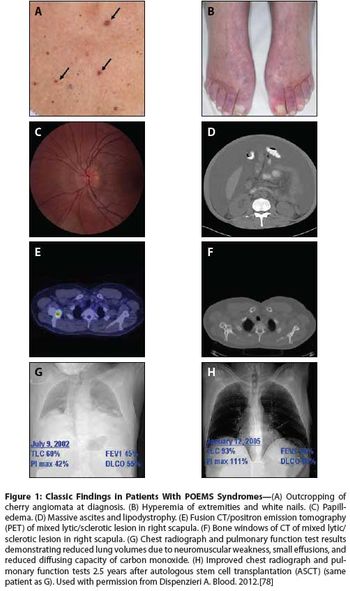
POEMS syndrome is a rare paraneoplastic syndrome that is caused by an underlying plasma cell disorder. Its main features include polyradiculoneuropathy, organomegaly, endocrinopathy, monoclonal plasma cell disorder, and skin changes.
Treatment with gemtuzumab ozogamicin improved the event-free survival in children and adolescents with acute myeloid leukemia by reducing the risk of relapse among those able to achieve remission, according to trial results presented at the 2013 ASH meeting.
Ponatinib showed significant antileukemic activity in patients with CML and ALL, according to a phase II study that included a wide range of disease stages and mutation status; patients in the trial had a relatively high rate of adverse thrombotic events, an issue which has led to recent regulatory controversy surrounding the drug.

Following recent trial data showing an increased risk of dangerous blood clots with ponatinib, which led the FDA to request that the manufacturer stop marketing the drug, the FDA’s European counterpart has adjusted its recommendations for ponatinib but has not changed its “positive opinion” that led to the approval.

This article reviews the etiology and incidence of renal adverse events in patients with multiple myeloma, the renal safety profile of single-agent carfilzomib from four phase II studies in patients with relapsed and/or refractory multiple myeloma, and the management of patients with multiple myeloma who receive carfilzomib and are at risk for renal complications.

This series of articles provides clinicians with a resource for the use of carfilzomib in the management of patients with multiple myeloma.

The adverse event profile of single-agent carfilzomib suggests that the agent is an important treatment option for patients with advanced multiple myeloma, particularly those with pre-existing peripheral neuropathy or those who are at risk for the development of peripheral neuropathy.

This article reviews the hematologic safety profile of carfilzomib in patients with relapsed/refractory multiple myeloma, as assessed in a cross-trial safety analysis of four phase II studies, and makes recommendations for the appropriate management of hematologic adverse events.

This article presents an overview of the cardiac and pulmonary safety profile of single-agent carfilzomib therapy in patients with relapsed and/or refractory multiple myeloma from an analysis of four phase II clinical studies, and provides practical recommendations for the management of patients at risk for cardiac events and pulmonary complication.
Patients able to achieve stringent complete response after undergoing autologous stem-cell transplantation for multiple myeloma achieved improved long-term outcomes, including overall survival and time to progression, compared with patients who achieved lesser levels of response.

Women with airborne allergies to plants, grass, and trees may have a moderate increased risk of developing blood cancers, according to a new study.

The outcome for patients with cryoglobulinemia has improved since the recognition that the condition is frequently associated with HCV and that elimination of this virus has therapeutic benefit for affected patients.

We recommend screening for cryoglobulinemia in all patients with HCV infection, livedo reticularis, vasculitic cutaneous ulcers, positive rheumatoid factor or rheumatoid vasculitis, membranoproliferative glomerulonephritis, or atypical Waldenström macroglobulinemia.
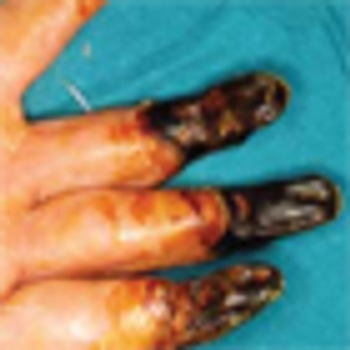
In spite of the complicated etiologic, clinical, and pathologic scenario of cryoglobulinemia, physicians can play a key role in its successful management by early recognition of the most common clinical presentations.

The FDA has approved ibrutinib (Imbruvica) for the treatment of patients with mantle-cell lymphoma (MCL), an aggressive and rare blood cancer.